Design and Baseline Characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease Trial
- PMID: 31655812
- PMCID: PMC6888890
- DOI: 10.1159/000503713
Design and Baseline Characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease Trial
Abstract
Background: Among diabetics, those with kidney disease have exceptionally high rates of cardiovascular (CV) morbidity and mortality, and progression of their underlying disease. Finerenone is a novel, non-steroidal, selective mineralocorticoid-receptor antagonist which has shown to reduce albuminuria in type 2 diabetes (T2D) patients with chronic kidney disease (CKD), while revealing only a low risk of hyperkalemia. However, the effect of finerenone on renal and CV outcomes has not been investigated in long-term trials yet.
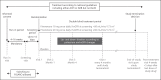
Methods: The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease -(FIDELIO-DKD) trial aims to assess the efficacy and safety of finerenone compared to placebo at reducing clinically important renal and CV outcomes in T2D patients with CKD. FIDELIO-DKD is a randomized, double-blind, placebo-controlled, parallel-group, event-driven trial running in 47 countries with an expected duration of approximately 5.5 years. FIDELIO-DKD randomized 5,734 patients with an estimated glomerular filtration rate (eGFR) ≥25-<75 mL/min/1.73 m2 and albuminuria (urinary albumin-to-creatinine ratio ≥30-≤5,000 mg/g). The study has at least 90% power to detect a 20% reduction in the risk of primary outcome (overall two-sided significance level α = 0.05), the composite of time to first occurrence of kidney failure, a sustained decrease of eGFR ≥40% from baseline over at least 4 weeks, or renal death.
Conclusion: FIDELIO-DKD will determine whether an optimally treated cohort of T2D patients with CKD at high risk of renal and CV events will experience cardiorenal benefits with the addition of finerenone to their treatment regimen.
Keywords: Aldosterone; Clinical; Diabetes; Kidney; Mineralocorticoid; Outcomes.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
G.L.B. reports research funding, paid to the University of Chicago Medicine, from Bayer, Janssen, and Vascular Dynamics; he acted as a consultant for Merck, Vascular Dynamics, Relypsa, Boehringer Ingelheim, Sanofi, Pfizer, Novo Nordisk, Ionis, and AstraZeneca; is an editor of American Journal of Nephrology, Nephrology, and Hypertension, and section editor of UpToDate; and is an associate editor of Diabetes Care and Hypertension Research. R.A. is a member of data safety monitoring committees for AstraZeneca and Ironwood Pharmaceuticals; a member of steering committees of randomized trials for Akebia, Bayer, Janssen, GlaxoSmithKline, Relypsa, and Sanofi and Genzyme US Companies; a member of adjudication committees for Bayer, Boehringer Ingelheim, and Janssen; and a member of a scientific advisory board or a consultant for Bird Rock Bio, Celgene, Daiichi Sankyo, Inc., Eli Lilly, Relypsa, Reata, Takeda Pharmaceuticals, USA, and ZS Pharma; he has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and he has received research grants from the U.S. Veterans Administration and the National Institutes of Health. S.D.A. has received research support from Abbott Vascular and Vifor International, and personal fees from Boehringer Ingelheim, Bayer, AstraZeneca, Novartis, Vifor International, Impulse Dynamics, Respicardia, and St. Jude Medical. B.P. reports consultant fees for Bayer, AstraZeneca, Sanofi, scPharmaceuticals, SQ Innovation, G3 Pharmaceuticals, Sarfez, Vifor, Cereno, Ardelyx, KBP Biosciences and Windtree Pharmaceuticals; he has stock options for KBP Biosciences, SQ Innovation, Sarfez, scPharmaceuticals, Cereno and G3 Pharmaceuticals, and Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9931412). L.M.R. has served as advisor/speaker for AstraZeneca, Bayer, Daiichi Sankyo, Medtronic, Novartis, and Recordati. C.N., P.K., and P.S. are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. A.C.F. is a full-time employee of Bayer S.A., Brazil. G.F. reported that he is a committee member of trials and registries sponsored by Bayer, Novartis, Servier, Vifor, Medtronic, and Boehringer Ingelheim.
Figures

References
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease a systematic review. Lancet. 2015 May;385((9981)):1975–82. - PubMed
-
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358((6)):580–91. - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001 Sep;345((12)):861–9. - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001 Sep;345((12)):851–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

