Catalytic Enantioselective Pyridine N-Oxidation
- PMID: 31656070
- PMCID: PMC6926419
- DOI: 10.1021/jacs.9b10414
Catalytic Enantioselective Pyridine N-Oxidation
Abstract
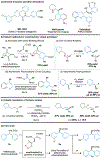
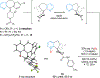
The catalytic, enantioselective N-oxidation of substituted pyridines is described. The approach is predicated on a biomolecule-inspired catalytic cycle wherein high levels of asymmetric induction are provided by aspartic-acid-containing peptides as the aspartyl side chain shuttles between free acid and peracid forms. Desymmetrizations of bis(pyridine) substrates bearing a remote pro-stereogenic center substituted with a group capable of hydrogen bonding to the catalyst are demonstrated. Our approach presents a new entry into chiral pyridine frameworks in a heterocycle-rich molecular environment. Representative functionalizations of the enantioenriched pyridine N-oxides further document the utility of this approach. Demonstration of the asymmetric N-oxidation in two venerable drug-like scaffolds, Loratadine and Varenicline, show the likely generality of the method for highly variable and distinct chiral environments, while also revealing that the approach is applicable to both pyridines and 1,4-pyrazines.
Figures




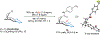

References
-
- Taylor RD; MacCoss M; Lawson ADG “Rings in Drugs” J. Med. Chem 2014, 57, 5845–5859. - PubMed
- Vitaku E; Smith DT; Njardarson JT “Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals” J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
- Henry GD “De novo Synthesis of Substituted Pyridines” Tetrahedron 2004, 60, 6043–6061.
- Hill MD “Recent Strategies for the Synthesis of Pyridine Derivatives” Chem. Eur. J 2010, 16, 12052–12062. - PubMed
-
-
For several representative examples of chiral pyridines in drugs and drug-like molecules, see:
- Roecker AJ; Mercer SP; Schreier JD; Cox CD; Fraley ME; Steen JT; Lemaire W; Bruno JG; Harrell CM; Garson SL; Gotter AL; Fox SV; Stevens J; Tannenbaum PL; Prueksaritanont T; Cabalu TD; Cui D; Stellabott J; Hartman GD; Young SD; Winrow CJ; Renger JJ; Coleman PJ “Discovery of 5′′- Chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3′′-terpyridine-3′-carboxamide (MK-1064): A Selective Orexin 2 Receptor Antagonist (2-SORA) for the Treatment of Insomnia” ChemMedChem 2014, 9, 311–322. - PubMed
- Ding J; Hall DG “Concise Synthesis and Antimalarial Activity of All Four Mefloquine Stereoisomers Using a Highly Enantioselective Catalytic Borylative Alkene Isomerization” Angew. Chem. Int. Ed 2013, 52, 8069–8073. - PubMed
- Rastelli EJ; Coltart DM “A Concise and Highly Enantioselective Total Synthesis of (+)-anti- and (−)-syn -Mefloquine Hydrochloride: Definitive Absolute Stereochemical Assignment of the Mefloquines” Angew. Chem. Int. Ed 2015, 54, 14070–14074. - PubMed
- Parton AH; Ali MH; Brookings DC; Brown JA; Ford DJ; Franklin RJ; Langham BJ; Neuss JC; Quincey JR “Quinoline and Quinoxaline Derivatives as Kinase Inhibitors” WO2012/032334 (2012).
- Brookings DC “8.15 - The Discovery and Development of Seletalisib: A Potent and Selective PI3Kδ Inhibitor for Inflammatory Diseases” In Comprehensive Medicinal Chemistry II, Chackalamannil S; Rotella D; Ward SE, Eds. Elsevier: Oxford, 2017; pp 366–407.
-
-
- Tanyeli C; Akhmedov İM; Işık M “Synthesis of Various Camphor-Based Chiral Pyridine Derivatives” Tetrahedron Lett 2004, 45, 5799–5801.
-
- Kuduk SD; DiPardo RM; Chang RK; Ng C; Bock MG “Reversal of Diastereoselection in the Addition of Grignard Reagents to Chiral 2-Pyridyl tert-Butyl (Ellman) Sulfinimines” Tetrahedron Lett 2004, 45, 6641–6643.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

