Toward the Optimization of (+)-[11C]PHNO Synthesis: Time Reduction and Process Validation
- PMID: 31656452
- PMCID: PMC6791232
- DOI: 10.1155/2019/4292596
Toward the Optimization of (+)-[11C]PHNO Synthesis: Time Reduction and Process Validation
Abstract
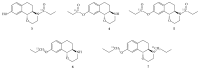
(+)-[11C]PHNO, a dopamine D2/3 receptor agonistic radiotracer, is applied for investigating the dopaminergic system via positron emission tomography (PET). An improved understanding of neuropsychiatric disorders associated with dysfunctions in the dopamine system and the underlying mechanism is a necessity in order to promote the development of new potential therapeutic drugs. In contrast to other broadly applied 11C-radiopharmaceuticals, the production of this radiotracer requires a challenging four-step radiosynthesis involving harsh reaction conditions and reactants as well as an inert atmosphere. Consequently, the production is prone to errors and troubleshooting after failed radiosyntheses remains time consuming. Hence, we aimed to optimize the radiosynthesis of (+)-[11C]PHNO for achieving better activity yields without loss of product quality. Therefore, we synthesized (+)-[11C]PHNO and omitted all heating and cooling steps leading to higher activity yields. As a result, radiosynthesis fully conducted at room temperature led to a time-reduced production procedure that saves about 5 min, which is an appreciable decay-prevention of around 15% of the activity yield. Additionally, we established a troubleshooting protocol by investigating reaction intermediates, byproducts, and impurities. Indeed, partial runs enabled the assignment of byproducts to their associated error source. Finally, we were able to generate a decision tree facilitating error detection in (+)-[11C]PHNO radiosynthesis.
Copyright © 2019 Sarah Pfaff et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures









References
-
- Breier A., Su T. P., Saunders R., et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences. 1997;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
