Stability of a monovalent rotavirus vaccine after exposure to different temperatures observed in KwaZulu-Natal, South Africa
- PMID: 31656482
- PMCID: PMC6794501
- DOI: 10.4314/ahs.v19i2.22
Stability of a monovalent rotavirus vaccine after exposure to different temperatures observed in KwaZulu-Natal, South Africa
Abstract
Background: Rotavirus infection and its associated hospitalization of children less than 5 years old in middle- and low-income countries remains a public health challenge. We hypothesized that the Rotarix®potency is affected by non-optimal temperatures which translates into reduced vaccine effectiveness in these settings.
Objective: To assess the effect of non-optimal temperatures on the potency of the Rotarix® vaccine in South Africa.
Methods: Rotarix® vaccine was exposed to temperatures reflecting breaches in the cold chain. Vero cells (ATCC CCL-81) grown in a 24-well tissue culture plates were infected with Rotarix® vaccine viruses after exposure to non-optimal temperatures and the potency of the vaccine was determined using the plaque assay.
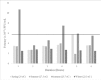
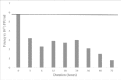
Results: Exposure of the Rotarix® vaccine to seasonal temperatures in KwaZulu-Natal for 6 hours and to extreme temperatures of 40oC for 72 hours as well as to -20°C and -80°C for 12 hours did not affect the potency of the vaccine beyond its expected standard of >7 x 105 PFU/ml.
Conclusion: This study revealed that the Rotarix® vaccine remains potent even after exposure to non-optimal temperatures. However, this study only explored the effect of a constant 'adverse' temperature on vaccine potency and not the effect of temperature fluctuations.
© 2019 Asowata et al.
References
-
- Mapaseka SL, Dewar JB, van der Merwe L, Geyer A, Tumbo J, Zweygarth M, et al. Prospective Hospital-Based Surveillance to Estimate Rotavirus Disease Burden in the Gauteng and North West Province of South Africa during 2003–2005. J Infect Dis [Internet] 2010 Sep 1;202(S1):S131–S138. [cited 2018 Oct 11]; Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/653558. - DOI - PubMed
-
- Steele AD, Peenze I, de Beer MC, Pager CT, Yeats J, Potgieter N, et al. Anticipating rotavirus vaccines: epidemiology and surveillance of rotavirus in South Africa. Vaccine [Internet] 2003 Jan 17;21(5–6):354–360. [cited 2018 Jan 22]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/12531632. - PubMed
-
- Steele AD, De Vos B, Tumbo J, Reynders J, Scholtz F, Bos P, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine [Internet] 2010 Sep 7;28(39):6542–6548. [cited 2018 Jan 22]; Available from: https://www.sciencedirect.com/science/article/pii/S0264410X08011328?via%.... - PubMed
-
- Steele AD, Glass R. Rotavirus in South Africa: From discovery to vaccine introduction. South African J Epidemiol Infect [Internet] 2011 Jan 15;26(4):184–190. [cited 2018 Jan 24]; Available from: https://www.tandfonline.com/doi/full/10.1080/10158782.2011.11441448. - DOI
-
- Groome M. Rotavirus vaccination in reducing diarrhoeal disease in Africa. [Internet] World Immunization week. 2015 Available from: www.nicd.ac.za/assets/files/Immunizationday2015_Groome.pdf.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources