Effects of different doses of erythropoietin in patients with myelodysplastic syndromes: A propensity score-matched analysis
- PMID: 31657156
- PMCID: PMC6912022
- DOI: 10.1002/cam4.2638
Effects of different doses of erythropoietin in patients with myelodysplastic syndromes: A propensity score-matched analysis
Abstract
Background: Erythropoiesis-stimulating agents effectively improve the hemoglobin levels in a fraction of anemic patients with myelodysplastic syndromes (MDS). Higher doses (HD) of recombinant human erythropoietin (rhEPO) have been proposed to overcome suboptimal response rates observed in MDS patients treated with lower "standard doses" (SD) of rhEPO. However, a direct comparison between the different doses of rhEPO is lacking.
Methods: A cohort of 104 MDS patients treated with HD was retrospectively compared to 208 patients treated with SD in a propensity score-matched analysis to evaluate hematological improvement-erythroid (HI-E) rate induced by the different doses of rhEPO. The impact of rhEPO doses on survival and progression to leukemia was also investigated.
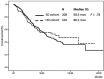
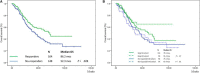
Results: Overall HI-E rate was 52.6%. No difference was observed between different rhEPO doses (P = .28) in matched cohorts; in a subgroup analysis, transfusion-dependent patients and patients with higher IPSS-R score obtained a higher HI-E rate with HD, although without significant impact on overall survival (OS). Achievement of HI-E resulted in superior OS. At univariate analysis, a higher HI-E rate was observed in transfusion-independent patients (P < .001), with a lower IPSS-R score (P < .001) and lower serum EPO levels (P = .027). Multivariate analysis confirmed that rhEPO doses were not significantly related to HI-E (P = .26). There was no significant difference in OS or progression to leukemia in patients treated with HD vs SD.
Conclusion: SD are substantially equally effective to HD to improve anemia and influencing survival in MDS patients stratified according to similar propensity to be exposed to rhEPO treatment.
Keywords: anemia; erythropoietin; myelodysplastic syndromes.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
EB served as a member of local advisory board for Janssen‐Cilag, Novartis, and Celgene. EA has received honoraria from Novartis and Celgene, involvement in local advisory boards for Jazz Pharmaceuticals and Roche and participation in DMC for Celgene and Vertex Pharmaceuticals Incorporated and CRISPR Therapeutics. DC has received honoraria from Novartis and Celgene. CF has received research funding, advisory committees, and speaker fees from Novartis, Janssen, and Celgene. PM received honoraria from and participation to advisory boards for Janssen e Amgen. ENO received research funding from Janssen‐Cilag. FP served as a member of advisory board for Novartis. VS has received honoraria from Celgene, Janssen, and Novartis. Advisory boards for Celgene, Janssen, Abbvie, Astex, Karyopharma, Acceleron. RAF, CS, BA, MB, TC, MC, MC, EC, ADC, PD, DF, DG, RML, EM, EM, MM, AP, FS, AS, MS, and RT have nothing to disclose.
Figures

References
-
- Santini V. Anemia as the main manifestation of myelodysplastic syndromes. Semin Hematol. 2015;52(4):348‐356. - PubMed
-
- Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594‐7603. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079‐2088. - PubMed
-
- Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175‐1178. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

