Inositol polyphosphate multikinase is a metformin target that regulates cell migration
- PMID: 31657647
- PMCID: PMC6894044
- DOI: 10.1096/fj.201900717RR
Inositol polyphosphate multikinase is a metformin target that regulates cell migration
Erratum in
-
Correction.FASEB J. 2020 Sep;34(9):13066-13068. doi: 10.1096/fsb2.20812. FASEB J. 2020. PMID: 33411330 Free PMC article. No abstract available.
Abstract
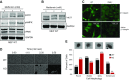
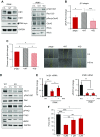
Metformin has been shown to alter cell adhesion protein expression, which is thought to play a role in its observed antitumor properties. We found that metformin treatment down-regulated integrin β1 concomitant with the loss of inositol polyphosphate multikinase (IPMK) in murine myocytes, adipocytes, and hepatocytes. To determine if IPMK was upstream of integrin β1 expression, we examined IPMK-/- mouse embryonic fibroblast cells and found that integrins β1 and β3 gene expression was reduced by half, relative to wild-type cells, whereas focal adhesion kinase (FAK) activity and Rho/Rac/Cdc42 protein levels were increased, resulting in migration defects. Using nanonet force microscopy, we determined that cell:extracellular matrix adhesion and cell contractility forces were decreased, confirming the functional relevance of integrin and Rho protein dysregulation. Pharmacological studies showed that inhibition of both FAK1 and proline-rich tyrosine kinase 2 partially restored integrin β1 expression, suggesting negative regulation of integrin β1 by FAK. Together our data indicate that IPMK participates in the regulation of cell migration and provides a potential link between metformin and wound healing impairment.-Tu-Sekine, B., Padhi, A., Jin, S., Kalyan, S., Singh, K., Apperson, M., Kapania, R., Hur, S. C., Nain, A., Kim, S. F. Inositol polyphosphate multikinase is a metformin target that regulates cell migration.
Keywords: adhesion; extracellular matrix; focal adhesion kinase; integrin; nanonet force microscopy.
Conflict of interest statement
The authors thank Jade West for assisting with cell tracking analyses, and Angie Chan for assisting with quantitative RT-PCR experiments (both from Johns Hopkins University). This work was supported by U.S. National Institutes of Health (NIH) Grant DA568921 (to S.F.K.), American Heart Association Grant 17SFRN33610014 (to S.F.K.), and National Science Foundation (NSF) Grants 1437101 and 1462916 (to A.N.). The authors declare no conflicts of interest.
Figures


References
-
- Schexnayder C., Broussard K., Onuaguluchi D., Poché A., Ismail M., McAtee L.F., Llopis S., Keizerweerd A., McFerrin H., Williams C. (2018) Metformin inhibits migration and invasion by suppressing ROS production and COX2 expression in MDA-MB-231 breast cancer cells. Int. J. Mol. Sci. 19, 1–13 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

