Selective Vulnerability of the Nucleus Basalis of Meynert Among Neuropathologic Subtypes of Alzheimer Disease
- PMID: 31657834
- PMCID: PMC6820048
- DOI: 10.1001/jamaneurol.2019.3606
Selective Vulnerability of the Nucleus Basalis of Meynert Among Neuropathologic Subtypes of Alzheimer Disease
Erratum in
-
Missing Funding Information in Funding/Support.JAMA Neurol. 2020 Feb 1;77(2):265. doi: 10.1001/jamaneurol.2019.4769. JAMA Neurol. 2020. PMID: 31904795 Free PMC article. No abstract available.
Abstract
Importance: Corticolimbic patterns of neurofibrillary tangle (NFT) accumulation define neuropathologic subtypes of Alzheimer disease (AD), which underlie the clinical heterogeneity observed antemortem. The cholinergic system, which is the target of acetylcholinesterase inhibitor therapy, is selectively vulnerable in AD.
Objective: To investigate the major source of cholinergic innervation, the nucleus basalis of Meynert (nbM), in order to determine whether there is differential involvement of NFT accumulation or neuronal loss among AD subtypes.
Design, setting, and participants: In this cross-sectional study, retrospective abstraction of clinical records and quantitative assessment of NFTs and neuron counts in the nbM was completed in January 2019 at the Mayo Clinic using the Florida Autopsied Multi-Ethnic (FLAME) cohort, which had been accessioned from 1991 until 2015. The FLAME cohort is derived from the deeded autopsy program funded throughout the State of Florida's memory disorder clinic referral services. Of the 2809 consecutively accessioned FLAME cohort, 1464 were identified as neuropathologically diagnosed AD cases and nondemented normal controls available for clinicopathologic assessment. Quantification of NFTs and neuronal density in the anterior nbM was performed blinded to neuropathologic groupings.
Main outcomes and measures: Demographic and clinical characteristics, including cognitive decline measured using the Mini-Mental State Examination score (range, 0-30), were evaluated. The anterior nbM was investigated quantitatively for neuronal loss and NFT accumulation.
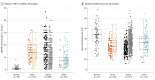
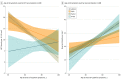
Results: In total, 1361 AD subtypes and 103 nondemented controls were assessed. The median (interquartile range) age at death was 72 (66-80) years in hippocampal sparing (HpSp) AD, 81 (76-86) years in typical AD, and 86 (82-90) years in limbic predominant AD. The median (interquartile range) count per 0.125 mm2 of thioflavin S-positive NFTs was highest in the nbM of HpSp AD (14 [9-20]; n = 163), lower in typical AD (10 [5-16]; n = 937), and lowest in limbic predominant AD (8 [5-11], n = 163) (P < .001). The median (interquartile range) neuronal density per millimeters squared was lowest in HpSp AD cases (22 [17-28]; n = 148), higher in typical AD (25 [19-30]; n = 727), and highest in limbic predominant AD (26 [19-32]; n = 127) (P = .002). Multivariable regression modeling of clinical and demographic variables was performed to assess overlap in NFT accumulation and neuronal density differences among AD subtypes. Higher NFT accumulation in the nbM was associated with younger age at onset for HpSp AD (β, -1.5; 95% CI, -2.9 to -0.15; P = .03) and typical AD (β, -3.2; 95% CI, -3.9 to -2.4; P < .001). In addition, higher NFT accumulation in the nbM of typical AD cases was associated with female sex (β, 2.5; 95% CI, 1.4-3.5; P < .001), apolipoprotein E ε4 allele (β, 1.3; 95% CI, 0.15-2.5; P = .03), and lower Mini-Mental State Examination scores (β, -1.8; 95% CI, -3.2 to -0.31; P = .02). Demographic and clinical progression variables were not associated with NFT accumulation in the nbM of limbic predominant AD cases.
Conclusions and relevance: These data provide supportive evidence that NFT accumulation in the nbM may underlie more widespread and severe cholinergic deficits in young-onset AD, in particular in patients with HpSp AD. Moreover, these findings underscore the importance of considering age at onset, sex, and apolipoprotein E genotype when assessing outcomes in AD.
Conflict of interest statement
Figures

References
-
- Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association . National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123(1):1-11. doi:10.1007/s00401-011-0910-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

