Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16
- PMID: 31657981
- PMCID: PMC7351342
- DOI: 10.1200/JCO.19.01692
Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16
Abstract
Purpose: Despite contemporary treatment, up to 10% of children with acute lymphoblastic leukemia still experience relapse. We evaluated whether a higher dosage of PEG-asparaginase and early intensification of triple intrathecal therapy would improve systemic and CNS control.
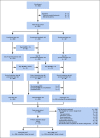
Patients and methods: Between 2007 and 2017, 598 consecutive patients age 0 to 18 years received risk-directed chemotherapy without prophylactic cranial irradiation in the St Jude Total Therapy Study 16. Patients were randomly assigned to receive PEG-asparaginase 3,500 U/m2 versus the conventional 2,500 U/m2. Patients presenting features that were associated with increased risk of CNS relapse received two extra doses of intrathecal therapy during the first 2 weeks of remission induction.
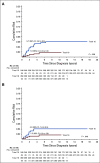
Results: The 5-year event-free survival and overall survival rates for the 598 patients were 88.2% (95% CI, 84.9% to 91.5%) and 94.1% (95% CI, 91.7% to 96.5%), respectively. Cumulative risk of any-isolated or combined-CNS relapse was 1.5% (95% CI, 0.5% to 2.5%). Higher doses of PEG-asparaginase did not affect treatment outcome. T-cell phenotype was the only independent risk factor for any CNS relapse (hazard ratio, 5.15; 95% CI, 1.3 to 20.6; P = . 021). Among 359 patients with features that were associated with increased risk for CNS relapse, the 5-year rate of any CNS relapse was significantly lower than that among 248 patients with the same features treated in the previous Total Therapy Study 15 (1.8% [95% CI, 0.4% to 3.3%] v 5.7% [95% CI, 2.8% to 8.6%]; P = .008). There were no significant differences in the cumulative risk of seizure or infection during induction between patients who did or did not receive the two extra doses of intrathecal treatment.
Conclusion: Higher doses of PEG-asparaginase failed to improve outcome, but additional intrathecal therapy during early induction seemed to contribute to improved CNS control without excessive toxicity for high-risk patients.
Trial registration: ClinicalTrials.gov NCT00274937.
Figures


References
-
- Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: Results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004) Lancet Oncol. 2009;10:957–966. - PubMed
-
- Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–615. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

