Infectious vaccine-derived rubella viruses emerge, persist, and evolve in cutaneous granulomas of children with primary immunodeficiencies
- PMID: 31658304
- PMCID: PMC6837625
- DOI: 10.1371/journal.ppat.1008080
Infectious vaccine-derived rubella viruses emerge, persist, and evolve in cutaneous granulomas of children with primary immunodeficiencies
Abstract
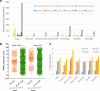
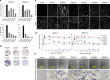
Rubella viruses (RV) have been found in an association with granulomas in children with primary immune deficiencies (PID). Here, we report the recovery and characterization of infectious immunodeficiency-related vaccine-derived rubella viruses (iVDRV) from diagnostic skin biopsies of four patients. Sequence evolution within PID hosts was studied by comparison of the complete genomic sequences of the iVDRVs with the genome of the vaccine virus RA27/3. The degree of divergence of each iVDRV correlated with the duration of persistence indicating continuous intrahost evolution. The evolution rates for synonymous and nonsynonymous substitutions were estimated to be 5.7 x 10-3 subs/site/year and 8.9 x 10-4 subs/site/year, respectively. Mutational spectra and signatures indicated a major role for APOBEC cytidine deaminases and a secondary role for ADAR adenosine deaminases in generating diversity of iVDRVs. The distributions of mutations across the genes and 3D hotspots for amino acid substitutions in the E1 glycoprotein identified regions that may be under positive selective pressure. Quasispecies diversity was higher in granulomas than in recovered infectious iVDRVs. Growth properties of iVDRVs were assessed in WI-38 fibroblast cultures. None of the iVDRV isolates showed complete reversion to wild type phenotype but the replicative and persistence characteristics of iVDRVs were different from those of the RA27/3 vaccine strain, making predictions of iVDRV transmissibility and teratogenicity difficult. However, detection of iVDRV RNA in nasopharyngeal specimen and poor neutralization of some iVDRV strains by sera from vaccinated persons suggests possible public health risks associated with iVDRV carriers. Detection of IgM antibody to RV in sera of two out of three patients may be a marker of virus persistence, potentially useful for identifying patients with iVDRV before development of lesions. Studies of the evolutionary dynamics of iVDRV during persistence will contribute to development of infection control strategies and antiviral therapies.
Conflict of interest statement
The authors have read the journal's policy and have the following conflicts: Andrey Zharkikh is an employee of Myriad Genetics. This company works in cancer genetics and diagnostics without any relation to pathogen studies and treatments. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures






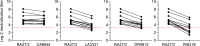
References
-
- Approved proposal. Animal ssRNA+ viruses. 2018.013S.R.Matonaviridae 2019. Available from: https://talk.ictvonline.org/files/ictv_official_taxonomy_updates_since_t....
-
- Rawls WE. Viral persistence in congenital rubella. Prog Med Virol. 1974;18:273–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

