Redox-Mediated Mechanism of Chemoresistance in Cancer Cells
- PMID: 31658599
- PMCID: PMC6826977
- DOI: 10.3390/antiox8100471
Redox-Mediated Mechanism of Chemoresistance in Cancer Cells
Abstract
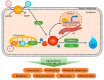
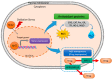
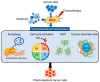
Cellular reactive oxygen species (ROS) status is stabilized by a balance of ROS generation and elimination called redox homeostasis. ROS is increased by activation of endoplasmic reticulum stress, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family members and adenosine triphosphate (ATP) synthesis of mitochondria. Increased ROS is detoxified by superoxide dismutase, catalase, and peroxiredoxins. ROS has a role as a secondary messenger in signal transduction. Cancer cells induce fluctuations of redox homeostasis by variation of ROS regulated machinery, leading to increased tumorigenesis and chemoresistance. Redox-mediated mechanisms of chemoresistance include endoplasmic reticulum stress-mediated autophagy, increased cell cycle progression, and increased conversion to metastasis or cancer stem-like cells. This review discusses changes of the redox state in tumorigenesis and redox-mediated mechanisms involved in tolerance to chemotherapeutic drugs in cancer.
Keywords: 5-Fluorouracil; antioxidant proteins; chemoresistance; oxaliplatin; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lu Q.B., Wan M.Y., Wang P.Y., Zhang C.X., Xu D.Y., Liao X., Sun H.J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFkappaB/mTOR/P70S6K signaling cascade. Redox Biol. 2018;14:656–668. doi: 10.1016/j.redox.2017.11.012. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

