Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines
- PMID: 31658607
- PMCID: PMC6829379
- DOI: 10.3390/ijms20205004
Chiral Cocrystal Solid Solutions, Molecular Complexes, and Salts of N-Triphenylacetyl-l-Tyrosine and Diamines
Abstract
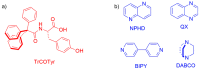
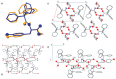
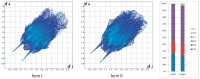
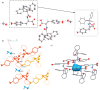
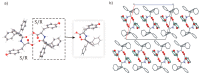
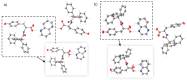
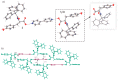
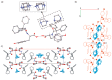
The molecular recognition process and the ability to form multicomponent supramolecular systems have been investigated for the amide of triphenylacetic acid and l-tyrosine (N-triphenylacetyl-l-tyrosine, TrCOTyr). The presence of several supramolecular synthons within the same amide molecule allows the formation of various multicomponent crystals, where TrCOTyr serves as a chiral host. Isostructural crystals of solvates with methanol and ethanol and a series of binary crystalline molecular complexes with selected organic diamines (1,5-naphthyridine, quinoxaline, 4,4'-bipyridyl, and DABCO) were obtained. The structures of the crystals were planned based on non-covalent interactions (O-H···N or N-H+···O- hydrogen bonds) present in a basic structural motif, which is a heterotrimeric building block consisting of two molecules of the host and one molecule of the guest. The complex of TrCOTyr with DABCO is an exception. The anionic dimers built off the TrCOTyr molecules form a supramolecular gutter, with trityl groups located on the edge and filled by DABCO cationic dimers. Whereas most of the racemic mixtures crystallize as racemic crystals or as conglomerates, the additional tests carried out for racemic N-triphenylacetyl-tyrosine (rac-TrCOTyr) showed that the compound crystallizes as a solid solution of enantiomers.
Keywords: amino acid; cocrystals; molecular complexes; solid solution; trityl group.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Steed J.W., Atwood J.L. Supramolecular Chemistry. Wiley-VCH; Weinheim, Germany: 2009.
-
- Fujita M. Molecular Self-Assembly Organic Versus Inorganic Approaches. Springer; Heidelberg, Germany: 2000.
-
- Lawrence D.S., Jiang T., Levett M. Self-Assembling Supramolecular Complexes. Chem. Rev. 1995;95:2229–2260. doi: 10.1021/cr00038a018. - DOI
-
- Lindoy L.F., Atkinson I.M. Self-Assembly in Supramolecular Systems. RSC; Cambridge, UK: 2000.
-
- MacGillivray L.R. Comprehensive Supramolecular Chemistry II. In: Atwood J.L., Gokel G.W., Barbour L.J., editors. Supramolecular Engineering: Designing The Solid State. Elsevier; Amsterdam, The Netherlands: 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

