Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections
- PMID: 31658632
- PMCID: PMC6843252
- DOI: 10.3390/microorganisms7100429
Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections
Abstract
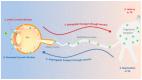
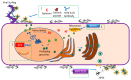
Herpes simplex virus type-1 (HSV-1) is a neurotropic, double-stranded DNA virus that can cause a wide variety of diseases, including many ocular pathologies. It is one of the leading causes of infectious blindness in the United States. Because of its ubiquitous nature and its potential to cause serious ocular maladies, there is a significant need for more effective antiviral therapies against ocular HSV-1. In this review, we discuss the lifecycle of HSV-1 as it pertains to corneal infections and the clinically approved as well as emerging treatments to combat HSV-1 infections. We also highlight some newly identified host targets for the antiviral drug development.
Keywords: acyclovir; antiviral; herpes simplex virus; herpesvirus; keratitis; ocular therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Whitley R.J. Herpesviruses. In: Baron S., editor. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

