The PAK1-Stat3 Signaling Pathway Activates IL-6 Gene Transcription and Human Breast Cancer Stem Cell Formation
- PMID: 31658701
- PMCID: PMC6826853
- DOI: 10.3390/cancers11101527
The PAK1-Stat3 Signaling Pathway Activates IL-6 Gene Transcription and Human Breast Cancer Stem Cell Formation
Abstract
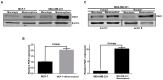
Cancer stem cells (CSCs) have unique properties, including self-renewal, differentiation, and chemoresistance. In this study, we found that p21-activated kinase (PAK1) inhibitor (Group I, PAK inhibitor, IPA-3) and inactivator (ivermectin) treatments inhibit cell proliferation and that tumor growth of PAK1-knockout cells in a mouse model is significantly reduced. IPA-3 and ivermectin inhibit CSC formation. PAK1 physically interacts with Janus Kinase 2 (JAK2), and JAK2 inhibitor (TG101209) treatment inhibits mammosphere formation and reduces the nuclear PAK1 protein level. PAK1 interacts with signal transducer and activator of transcription 3 (Stat3), and PAK1 and Stat3 colocalize in the nucleus. We show through electrophoretic mobility shift assay (EMSA), chromatin immunoprecipitation (ChIP), and reporter assays that the PAK1/Stat3 complex binds to the IL-6 promoter and regulates the transcription of the IL-6 gene. Inhibition of PAK1 and JAK2 in mammospheres reduces the nuclear pStat3 and extracellular IL-6 levels. PAK1 inactivation inhibits CSC formation by decreasing pStat3 and extracellular IL-6 levels. Our results reveal that JAK2/PAK1 dysregulation inhibits the Stat3 signaling pathway and CSC formation, the PAK1/Stat3 complex regulates IL-6 gene expression, PAK1/Stat3 signaling regulates CSC formation, and PAK1 may be an important target for treating breast cancer.
Keywords: IL-6; JAK2; PAK1; Stat3; cancer stem cell; mammosphere.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Marotta L.L., Almendro V., Marusyk A., Shipitsin M., Schemme J., Walker S.R., Bloushtain-Qimron N., Kim J.J., Choudhury S.A., Maruyama R., et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011;121:2723–2735. doi: 10.1172/JCI44745. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

