Recent Advances in Skin Chemical Sensors
- PMID: 31658706
- PMCID: PMC6832670
- DOI: 10.3390/s19204376
Recent Advances in Skin Chemical Sensors
Abstract
This review summarizes the latest developments in the field of skin chemical sensors, in particular wearable ones. Five major applications are covered in the present work: (i) sweat analysis, (ii) skin hydration, (iii) skin wounds, (iv) perspiration of volatile organic compounds, and (v) general skin conditions. For each application, the detection of the most relevant analytes is described in terms of transduction principles and sensor performances. Special attention is paid to the biological fluid collection and storage and devices are also analyzed in terms of reusability and lifetime. This review highlights the existing gaps between current performances and those needed to promote effective commercialization of sensors; future developments are also proposed.
Keywords: diabetes; flexible; printed electronics; skin sensors; smart dressings; smart tattoos; sweat; wearable.
Conflict of interest statement
The author declares no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data and in the writing of the manuscript.
Figures






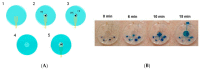






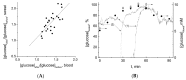


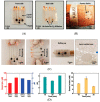

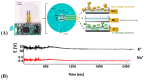
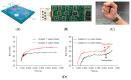



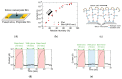
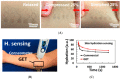




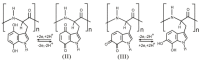









References
-
- Almansoori M.T., Li X., Zheng L. A Brief Review on E-skin and its Multifunctional Sensing Applications. Curr. Smart Mater. 2019;4:3–14. doi: 10.2174/2405465804666190313154903. - DOI
-
- Amjadi M., Kyung K.U., Park I., Sitti M., Kyung K.U., Kyung K. Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review. Adv. Funct. Mater. 2016;26:1678–1698. doi: 10.1002/adfm.201504755. - DOI
-
- Lou Z., Chen S., Wang L., Shi R., Li L., Jiang K., Chen D., Shen G. Ultrasensitive and ultraflexible e-skins with dual functionalities for wearable electronics. Nano Energy. 2017;38:28–35. doi: 10.1016/j.nanoen.2017.05.024. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

