Stapled Peptides-A Useful Improvement for Peptide-Based Drugs
- PMID: 31658723
- PMCID: PMC6832507
- DOI: 10.3390/molecules24203654
Stapled Peptides-A Useful Improvement for Peptide-Based Drugs
Abstract
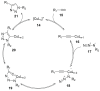
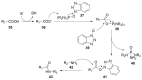
Peptide-based drugs, despite being relegated as niche pharmaceuticals for years, are now capturing more and more attention from the scientific community. The main problem for these kinds of pharmacological compounds was the low degree of cellular uptake, which relegates the application of peptide-drugs to extracellular targets. In recent years, many new techniques have been developed in order to bypass the intrinsic problem of this kind of pharmaceuticals. One of these features is the use of stapled peptides. Stapled peptides consist of peptide chains that bring an external brace that force the peptide structure into an α -helical one. The cross-link is obtained by the linkage of the side chains of opportune-modified amino acids posed at the right distance inside the peptide chain. In this account, we report the main stapling methodologies currently employed or under development and the synthetic pathways involved in the amino acid modifications. Moreover, we report the results of two comparative studies upon different kinds of stapled-peptides, evaluating the properties given from each typology of staple to the target peptide and discussing the best choices for the use of this feature in peptide-drug synthesis.
Keywords: cellular uptake; helicity; peptide drugs; stapled peptide; structurally constrained peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









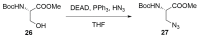


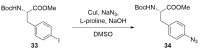










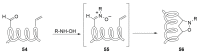





References
-
- Nelson D.L., Cox M.M. Lehninger’s Principles of Biochemistry. 4th ed. W. H. Freeman and Company; New York, NY, USA: 2005.
-
- Osborne T.B. The Vegetable Protein. Longman Harlow; London, UK: 1909.
-
- Mulder G.J. Sur la composition de quelques substances animales. Bull. Sci. Phys. Nat. Neerl. 1838;1:104–119.
-
- Harold H. Origin of the Word ‘Protein’. Nature. 1951;168:244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

