Analysis of Procollagen C-Proteinase Enhancer-1/Glycosaminoglycan Binding Sites and of the Potential Role of Calcium Ions in the Interaction
- PMID: 31658765
- PMCID: PMC6829435
- DOI: 10.3390/ijms20205021
Analysis of Procollagen C-Proteinase Enhancer-1/Glycosaminoglycan Binding Sites and of the Potential Role of Calcium Ions in the Interaction
Abstract
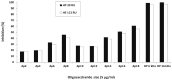
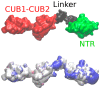
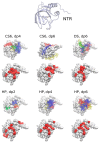
In this study, we characterize the interactions between the extracellular matrix protein, procollagen C-proteinase enhancer-1 (PCPE-1), and glycosaminoglycans (GAGs), which are linear anionic periodic polysaccharides. We applied molecular modeling approaches to build a structural model of full-length PCPE-1, which is not experimentally available, to predict GAG binding poses for various GAG lengths, types and sulfation patterns, and to determine the effect of calcium ions on the binding. The computational data are analyzed and discussed in the context of the experimental results previously obtained using surface plasmon resonance binding assays. We also provide experimental data on PCPE-1/GAG interactions obtained using inhibition assays with GAG oligosaccharides ranging from disaccharides to octadecasaccharides. Our results predict the localization of GAG-binding sites at the amino acid residue level onto PCPE-1 and is the first attempt to describe the effects of ions on protein-GAG binding using modeling approaches. In addition, this study allows us to get deeper insights into the in silico methodology challenges and limitations when applied to GAG-protein interactions.
Keywords: calcium ions; computational analysis of protein-glycosaminoglycan interactions; fragment-based docking; glycosaminoglycans; procollagen C-proteinase enhancer-1.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Esko J.D., Kimata K., Lindahl U. Proteoglycans and Sulfated Glycosaminoglycans. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., Hart G.W., Etzler M.E., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2009. pp. 1–784. - PubMed
-
- Shute J. Glycosaminoglycan and chemokine/growth factor interactions. Handb. Exp. Pharmacol. 2012;207:307–324. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

