Modeling the dynamics of Plasmodium falciparum gametocytes in humans during malaria infection
- PMID: 31658944
- PMCID: PMC6819085
- DOI: 10.7554/eLife.49058
Modeling the dynamics of Plasmodium falciparum gametocytes in humans during malaria infection
Abstract
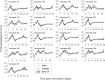
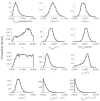
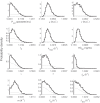
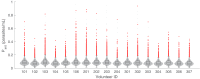
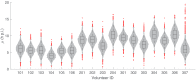
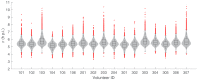
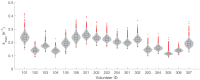
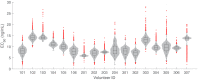
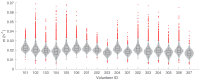
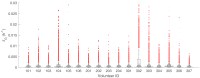
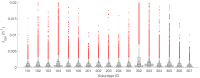
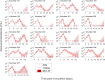
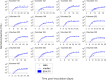
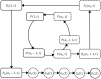
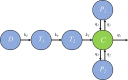
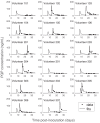
Renewed efforts to eliminate malaria have highlighted the potential to interrupt human-to-mosquito transmission - a process mediated by gametocyte kinetics in human hosts. Here we study the in vivo dynamics of Plasmodium falciparum gametocytes by establishing a framework which incorporates improved measurements of parasitemia, a novel gametocyte dynamics model and model fitting using Bayesian hierarchical inference. We found that the model provides an excellent fit to the clinical data from 17 volunteers infected with P. falciparum (3D7 strain) and reliably predicts observed gametocytemia. We estimated the sexual commitment rate and gametocyte sequestration time to be 0.54% (95% credible interval: 0.30-1.00%) per asexual replication cycle and 8.39 (6.54-10.59) days respectively. We used the data-calibrated model to investigate human-to-mosquito transmissibility, providing a method to link within-human host infection kinetics to epidemiological-scale infection and transmission patterns.
Keywords: P. falciparum; bayesian inference; computational biology; epidemiology; gametocyte dynamics; global health; mathematical modelling; systems biology.
© 2019, Cao et al.
Conflict of interest statement
PC, KC, SZ, TW, JT, JS, JM, JM No competing interests declared
Figures





References
-
- Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. PNAS. 2011;108:E1214–E1223. doi: 10.1073/pnas.1112037108. - DOI - PMC - PubMed
-
- Bolscher JM, Koolen KMJ, van Gemert GJ, van de Vegte-Bolmer MG, Bousema T, Leroy D, Sauerwein RW, Dechering KJ. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. Journal of Antimicrobial Chemotherapy. 2015;70:1357–1366. doi: 10.1093/jac/dkv003. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- Discovery project DP170103076/Australian Research Council/International
- 1025319/National Health and Medical Research Council/International
- Centre for Research Excellence 1134989/National Health and Medical Research Council/International
- Early Career Researcher Award DE170100785/Australian Research Council/International
- Senior Research Fellowship 1104975/National Health and Medical Research Council/International
LinkOut - more resources
Full Text Sources

