Human-specific tandem repeat expansion and differential gene expression during primate evolution
- PMID: 31659027
- PMCID: PMC6859368
- DOI: 10.1073/pnas.1912175116
Human-specific tandem repeat expansion and differential gene expression during primate evolution
Abstract
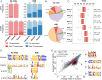
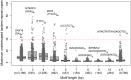
Short tandem repeats (STRs) and variable number tandem repeats (VNTRs) are important sources of natural and disease-causing variation, yet they have been problematic to resolve in reference genomes and genotype with short-read technology. We created a framework to model the evolution and instability of STRs and VNTRs in apes. We phased and assembled 3 ape genomes (chimpanzee, gorilla, and orangutan) using long-read and 10x Genomics linked-read sequence data for 21,442 human tandem repeats discovered in 6 haplotype-resolved assemblies of Yoruban, Chinese, and Puerto Rican origin. We define a set of 1,584 STRs/VNTRs expanded specifically in humans, including large tandem repeats affecting coding and noncoding portions of genes (e.g., MUC3A, CACNA1C). We show that short interspersed nuclear element-VNTR-Alu (SVA) retrotransposition is the main mechanism for distributing GC-rich human-specific tandem repeat expansions throughout the genome but with a bias against genes. In contrast, we observe that VNTRs not originating from retrotransposons have a propensity to cluster near genes, especially in the subtelomere. Using tissue-specific expression from human and chimpanzee brains, we identify genes where transcript isoform usage differs significantly, likely caused by cryptic splicing variation within VNTRs. Using single-cell expression from cerebral organoids, we observe a strong effect for genes associated with transcription profiles analogous to intermediate progenitor cells. Finally, we compare the sequence composition of some of the largest human-specific repeat expansions and identify 52 STRs/VNTRs with at least 40 uninterrupted pure tracts as candidates for genetically unstable regions associated with disease.
Keywords: STR; VNTR; genome instability; tandem repeat; tandem repeat expansion.
Conflict of interest statement
Competing interest statement: E.E.E. is on the scientific advisory board of DNAnexus, Inc.
Figures
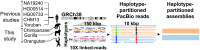


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

