Microbial communities in the tropical air ecosystem follow a precise diel cycle
- PMID: 31659049
- PMCID: PMC6859341
- DOI: 10.1073/pnas.1908493116
Microbial communities in the tropical air ecosystem follow a precise diel cycle
Abstract
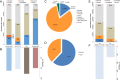
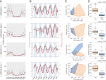
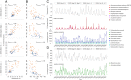
The atmosphere is vastly underexplored as a habitable ecosystem for microbial organisms. In this study, we investigated 795 time-resolved metagenomes from tropical air, generating 2.27 terabases of data. Despite only 9 to 17% of the generated sequence data currently being assignable to taxa, the air harbored a microbial diversity that rivals the complexity of other planetary ecosystems. The airborne microbial organisms followed a clear diel cycle, possibly driven by environmental factors. Interday taxonomic diversity exceeded day-to-day and month-to-month variation. Environmental time series revealed the existence of a large core of microbial taxa that remained invariable over 13 mo, thereby underlining the long-term robustness of the airborne community structure. Unlike terrestrial or aquatic environments, where prokaryotes are prevalent, the tropical airborne biomass was dominated by DNA from eukaryotic phyla. Specific fungal and bacterial species were strongly correlated with temperature, humidity, and CO2 concentration, making them suitable biomarkers for studying the bioaerosol dynamics of the atmosphere.
Keywords: air microbiome; bioaerosols; microbial ecology; temperature; tropics.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Delort A. M., et al. , A short overview of the microbial population in clouds: Potential roles in atmospheric chemistry and nucleation processes. Atmos. Res. 98, 249–260 (2010).
-
- Smith D. J., et al. , Free tropospheric transport of microorganisms from Asia to North America. Microb. Ecol. 64, 973–985 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

