On the mechanism of GIRK2 channel gating by phosphatidylinositol bisphosphate, sodium, and the Gβγ dimer
- PMID: 31659119
- PMCID: PMC6901319
- DOI: 10.1074/jbc.RA119.010047
On the mechanism of GIRK2 channel gating by phosphatidylinositol bisphosphate, sodium, and the Gβγ dimer
Abstract
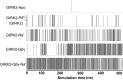
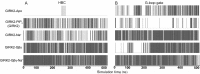
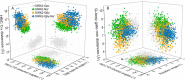
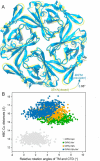
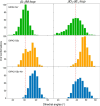
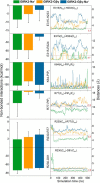
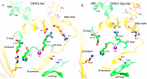
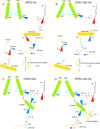
G protein-gated inwardly rectifying K+ (GIRK) channels belong to the inward-rectifier K+ (Kir) family, are abundantly expressed in the heart and the brain, and require that phosphatidylinositol bisphosphate is present so that intracellular channel-gating regulators such as Gβγ and Na+ ions can maintain the channel-open state. However, despite high-resolution structures (GIRK2) and a large number of functional studies, we do not have a coherent picture of how Gβγ and Na+ ions control gating of GIRK2 channels. Here, we utilized computational modeling and all-atom microsecond-scale molecular dynamics simulations to determine which gates are controlled by Na+ and Gβγ and how each regulator uses the channel domain movements to control gate transitions. We found that Na+ ions control the cytosolic gate of the channel through an anti-clockwise rotation, whereas Gβγ stabilizes the transmembrane gate in the open state through a rocking movement of the cytosolic domain. Both effects alter the way in which the channel interacts with phosphatidylinositol bisphosphate and thereby stabilizes the open states of the respective gates. These studies of GIRK channel dynamics present for the first time a comprehensive structural model that is consistent with the great body of literature on GIRK channel function.
Keywords: G protein; GIRK2 channel; Gβγ dimer; PIP2; allosteric regulation; channel activation; gating; intracellular Na+; membrane protein; molecular dynamics; molecular dynamics simulations; molecular modeling; phosphoinositide; potassium channel; transmembrane domain.
© 2019 Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

