Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway
- PMID: 31659157
- PMCID: PMC6817826
- DOI: 10.1038/s41419-019-2055-6
Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway
Abstract
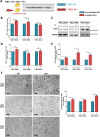
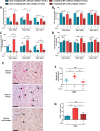
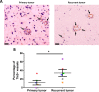
Glioblastomas (GBM) are brain tumors with a poor prognosis despite treatment that combines surgical resection and radio-chemotherapy. These tumors are characterized by abundant vascularization and significant cellular heterogeneity including GBM stem-like cells (GSC) which contribute to tumor aggressiveness, resistance, and recurrence. Recent data has demonstrated that GSC are directly involved in the formation of new vessels via their transdifferentiation into Tumor Derived Endothelial Cells (TDEC). We postulate that cellular stress such as ionizing radiation (IR) could enhance the transdifferentiation of GSC into TDEC. GSC neurospheres isolated from 3 different patients were irradiated or not and were then transdifferentiated into TDEC. In fact, TDEC obtained from irradiated GSC (TDEC IR+) migrate more towards VEGF, form more pseudotubes in MatrigelTM in vitro and develop more functional blood vessels in MatrigelTM plugs implanted in Nude mice than TDEC obtained from non-irradiated GSC. Transcriptomic analysis allows us to highlight an overexpression of Tie2 in TDEC IR+. All IR-induced effects on TDEC were abolished by using a Tie2 kinase inhibitor, which confirms the role of the Tie2 signaling pathway in this process. Finally, by analyzing Tie2 expression in patient GBMs by immunohistochemistry, we demonstrated that the number of Tie2+ vessels increases in recurrent GBM compared with matched untreated tumors. In conclusion, we demonstrate that IR potentiates proangiogenic features of TDEC through the Tie2 signaling pathway, which indicates a new pathway of treatment-induced tumor adaptation. New therapeutic strategies that associate standard treatment and a Tie2 signaling pathway inhibitor should be considered for future trials.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Louis, D., Ohgazi, H., Wiestler, O. & Cavenee, W. Diffuse astrocytic and oligodendroglial tumours. World Health Organization Histological Classifications of Tumours of the Central Nervous System. (4th ed., Revised. Lyon: International Agency for Research Centre; 2016).
-
- Stupp, R., Hegi, M. E. & Mason, W. P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

