Auger electrons for cancer therapy - a review
- PMID: 31659527
- PMCID: PMC6800417
- DOI: 10.1186/s41181-019-0075-2
Auger electrons for cancer therapy - a review
Abstract
Background: Auger electrons (AEs) are very low energy electrons that are emitted by radionuclides that decay by electron capture (e.g. 111In, 67Ga, 99mTc, 195mPt, 125I and 123I). This energy is deposited over nanometre-micrometre distances, resulting in high linear energy transfer (LET) that is potent for causing lethal damage in cancer cells. Thus, AE-emitting radiotherapeutic agents have great potential for treatment of cancer. In this review, we describe the radiobiological properties of AEs, their radiation dosimetry, radiolabelling methods, and preclinical and clinical studies that have been performed to investigate AEs for cancer treatment.
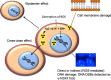
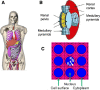
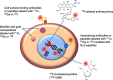
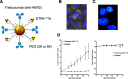
Results: AEs are most lethal to cancer cells when emitted near the cell nucleus and especially when incorporated into DNA (e.g. 125I-IUdR). AEs cause DNA damage both directly and indirectly via water radiolysis. AEs can also kill targeted cancer cells by damaging the cell membrane, and kill non-targeted cells through a cross-dose or bystander effect. The radiation dosimetry of AEs considers both organ doses and cellular doses. The Medical Internal Radiation Dose (MIRD) schema may be applied. Radiolabelling methods for complexing AE-emitters to biomolecules (antibodies and peptides) and nanoparticles include radioiodination (125I and 123I) or radiometal chelation (111In, 67Ga, 99mTc). Cancer cells exposed in vitro to AE-emitting radiotherapeutic agents exhibit decreased clonogenic survival correlated at least in part with unrepaired DNA double-strand breaks (DSBs) detected by immunofluorescence for γH2AX, and chromosomal aberrations. Preclinical studies of AE-emitting radiotherapeutic agents have shown strong tumour growth inhibition in vivo in tumour xenograft mouse models. Minimal normal tissue toxicity was found due to the restricted toxicity of AEs mostly on tumour cells targeted by the radiotherapeutic agents. Clinical studies of AEs for cancer treatment have been limited but some encouraging results were obtained in early studies using 111In-DTPA-octreotide and 125I-IUdR, in which tumour remissions were achieved in several patients at administered amounts that caused low normal tissue toxicity, as well as promising improvements in the survival of glioblastoma patients with 125I-mAb 425, with minimal normal tissue toxicity.
Conclusions: Proof-of-principle for AE radiotherapy of cancer has been shown preclinically, and clinically in a limited number of studies. The recent introduction of many biologically-targeted therapies for cancer creates new opportunities to design novel AE-emitting agents for cancer treatment. Pierre Auger did not conceive of the application of AEs for targeted cancer treatment, but this is a tremendously exciting future that we and many other scientists in this field envision.
Keywords: 111In; Auger electrons; Cancer treatment; Clinical studies; Dosimetry; Monoclonal antibodies; Nanoparticles; Peptides; Preclinical studies; Radiolabelling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- 65-Terbium-161. National Nuclear Data Centre. (2011).https://www.nndc.bnl.gov/mird/.
-
- Abuqbeitah M, Demir M, Çavdar İ, Tanyildizi H, Yeyin N, Uslu-Beşli L, Kabasakal L, Işıkcı Nİ, Sönmezoğlu K. Red bone marrow dose estimation using several internal dosimetry models for prospective dosimetry-oriented radioiodine therapy. Radiat Environ Biophys. 2018;57(4):395–404. - PubMed
-
- Aghevlian S, Lu Y, Winnik MA, Hedley DW, Reilly RM. Panitumumab modified with metal-chelating polymers (MCP) complexed to 111In and 177Lu—an EGFR-targeted theranostic for pancreatic cancer. Mol Pharm. 2018;15(3):1150–1159. - PubMed
-
- Areberg J, Björkman S, Einarsson L, Frankenberg B, Lundqvist H, Mattsson S, Norrgren K, Scheike O, Wallin R. Gamma camera imaging of platinum in tumours and tissues of patients after administration of 191Pt-cisplatin. Acta Oncol. 1999;38(2):221–228. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
