Production and characterization of no-carrier-added 161Tb as an alternative to the clinically-applied 177Lu for radionuclide therapy
- PMID: 31659528
- PMCID: PMC6620226
- DOI: 10.1186/s41181-019-0063-6
Production and characterization of no-carrier-added 161Tb as an alternative to the clinically-applied 177Lu for radionuclide therapy
Abstract
Background: 161Tb is an interesting radionuclide for cancer treatment, showing similar decay characteristics and chemical behavior to clinically-employed 177Lu. The therapeutic effect of 161Tb, however, may be enhanced due to the co-emission of a larger number of conversion and Auger electrons as compared to 177Lu. The aim of this study was to produce 161Tb from enriched 160Gd targets in quantity and quality sufficient for first application in patients.
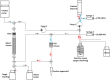
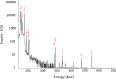
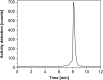
Methods: No-carrier-added 161Tb was produced by neutron irradiation of enriched 160Gd targets at nuclear research reactors. The 161Tb purification method was developed with the use of cation exchange (Sykam resin) and extraction chromatography (LN3 resin), respectively. The resultant product (161TbCl3) was characterized and the 161Tb purity compared with commercial 177LuCl3. The purity of the final product (161TbCl3) was analyzed by means of γ-ray spectrometry (radionuclidic purity) and radio TLC (radiochemical purity). The radiolabeling yield of 161Tb-DOTA was assessed over a two-week period post processing in order to observe the quality change of the obtained 161Tb towards future clinical application. To understand how the possible drug products (peptides radiolabeled with 161Tb) vary with time, stability of the clinically-applied somatostatin analogue DOTATOC, radiolabeled with 161Tb, was investigated over a 24-h period. The radiolytic stability experiments were compared to those performed with 177Lu-DOTATOC in order to investigate the possible influence of conversion and Auger electrons of 161Tb on peptide disintegration.
Results: Irradiations of enriched 160Gd targets yielded 6-20 GBq 161Tb. The final product was obtained at an activity concentration of 11-21 MBq/μL with ≥99% radionuclidic and radiochemical purity. The DOTA chelator was radiolabeled with 161Tb or 177Lu at the molar activity deemed useful for clinical application, even at the two-week time point after end of chemical separation. DOTATOC, radiolabeled with either 161Tb or 177Lu, was stable over 24 h in the presence of a stabilizer.
Conclusions: In this study, it was shown that 161Tb can be produced in high activities using different irradiation facilities. The developed method for 161Tb separation from the target material yielded 161TbCl3 in quality suitable for high-specific radiolabeling, relevant for future clinical application.
Keywords: 161Tb; Auger/conversion electrons; DOTA; Purification method; Somatostatin analogues.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Comment in
-
Opportunities and potential challenges of using terbium-161 for targeted radionuclide therapy in clinics.Eur J Nucl Med Mol Imaging. 2023 Sep;50(11):3181-3184. doi: 10.1007/s00259-023-06316-y. Eur J Nucl Med Mol Imaging. 2023. PMID: 37436459 No abstract available.
References
-
- Brezovcsik K. Separation of radioactive terbium from massive Gd targets for medical use. J Radioanal Nucl Chem. 2018;316:775–780. doi: 10.1007/s10967-018-5718-3. - DOI
-
- CHMP . Assessment report EndolucinBeta. 2016. pp. 30–37.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
