Mechanisms of Human Immunodeficiency Virus-Associated Lymphocyte Regulated Cell Death
- PMID: 31659912
- PMCID: PMC7044792
- DOI: 10.1089/AID.2019.0213
Mechanisms of Human Immunodeficiency Virus-Associated Lymphocyte Regulated Cell Death
Abstract
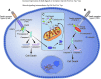
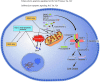
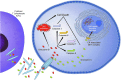
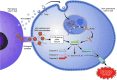
Human immunodeficiency virus-1 (HIV-1) causes CD4 T cell depletion through a number of mechanisms, including programmed cell death pathways (both apoptotic and nonapoptotic). In the setting of HIV-1 infection, the enhanced lymphocyte cell death occurs as a consequence of complex interactions between the host immune system and viral factors, which are reviewed herein. On the other hand, the main challenge to HIV-1 eradication is the development of latent infection in a subset of long lived cells, including CD4+ T cells and macrophages, which resist HIV-induced cell death. Understanding the potential mechanisms of how HIV-1 induces lymphocyte cell death is critical to the "kick and kill" cure strategy, which relies on the effective killing of reactivated, HIV-1-infected cells.
Keywords: apoptosis; autophagy; human immunodeficiency virus; necroptosis.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS: Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science 1984;225:840–842 - PubMed
-
- Laurent-Crawford AG, Krust B, Muller S, et al. : The cytopathic effect of HIV is associated with apoptosis. Virology 1991;185:829–839 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

