Clinical application of injectable growth factor for bone regeneration: a systematic review
- PMID: 31660090
- PMCID: PMC6805537
- DOI: 10.1186/s41232-019-0109-x
Clinical application of injectable growth factor for bone regeneration: a systematic review
Abstract
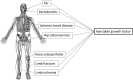
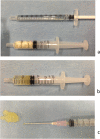
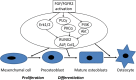
Bone regeneration has been the ultimate goal in the field of bone and joint medicine and has been evaluated through various basic research studies to date. Translational research of regenerative medicine has focused on three primary approaches, which are expected to increase in popularity: cell therapy, proteins, and artificial materials. Among these, the local injection of a gelatin hydrogel impregnated with the protein fibroblast growth factor (FGF)-2 is a biomaterial technique that has been developed in Japan. We have previously reported the efficacy of gelatin hydrogel containing injectable FGF-2 for the regenerative treatment of osteonecrosis of the femoral head. Injectable growth factors will probably be developed in the future and gain popularity as a medical approach in various fields as well as orthopedics. Several clinical trials have already been conducted and have focused on this technique, reporting its efficacy and safety. To date, reports of the clinical application of FGF-2 in revascularization for critical limb ischemia, treatment of periodontal disease, early bone union for lower limb fracture and knee osteotomy, and bone regeneration for osteonecrosis of the femoral head have been based on basic research conducted in Japan. In the present report, we present an extensive review of clinical applications using injectable growth factors and discuss the associated efficacy and safety of their administration.
Keywords: Bone regeneration; Cell proliferation; Clinical trial; Drug delivery system; Fibroblast growth factor; Gelatin hydrogel; Growth factor; Osteonecrosis; Tissue engineering.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

