A Muscle Hybrid Promoter as a Novel Tool for Gene Therapy
- PMID: 31660418
- PMCID: PMC6807297
- DOI: 10.1016/j.omtm.2019.09.001
A Muscle Hybrid Promoter as a Novel Tool for Gene Therapy
Abstract
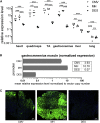
Gene therapy is a promising strategy to cure rare diseases. The lack of regulatory sequences ensuring specific and robust expression in skeletal and cardiac muscle is a substantial limitation of gene therapy efficiency targeting the muscle tissue. Here we describe a novel muscle hybrid (MH) promoter that is highly active in both skeletal and cardiac muscle cells. It has an easily exchangeable modular structure, including an intronic module that highly enhances the expression of the gene driven by it. In cultured myoblasts, myotubes, and cardiomyocytes, the MH promoter gives relatively stable expression as well as higher activity and protein levels than the standard CMV and desmin gene promoters or the previously developed synthetic or CKM-based promoters. Combined with AAV2/9, the MH promoter also provides a high in vivo expression level in skeletal muscle and the heart after both intramuscular and systemic delivery. It is much more efficient than the desmin-encoding gene promoter, and it maintains the same specificity. This novel promoter has potential for gene therapy in muscle cells. It can provide stable transgene expression, ensuring high levels of therapeutic protein, and limited side effects because of its specificity. This constitutes an improvement in the efficiency of genetic disease therapy.
Keywords: AAV; C2C12; expression cassette; heart; muscle promoter; myotubes; skeletal muscles; vector.
© 2019 The Authors.
Figures





References
-
- Theadom A., Rodrigues M., Roxburgh R., Balalla S., Higgins C., Bhattacharjee R., Jones K., Krishnamurthi R., Feigin V. Prevalence of muscular dystrophies: a systematic literature review. Neuroepidemiology. 2014;43:259–268. - PubMed
-
- Chuah M.K., Collen D., VandenDriessche T. Gene therapy for hemophilia. J. Gene Med. 2001;3:3–20. - PubMed
-
- Bohl D., Heard J.M. Delivering erythropoietin through genetically engineered cells. J. Am. Soc. Nephrol. 2000;11(Suppl 16):S159–S162. - PubMed
-
- Kreiss P., Bettan M., Crouzet J., Scherman D. Erythropoietin secretion and physiological effect in mouse after intramuscular plasmid DNA electrotransfer. J. Gene Med. 1999;1:245–250. - PubMed
-
- MacColl G.S., Novo F.J., Marshall N.J., Waters M., Goldspink G., Bouloux P.M. Optimisation of growth hormone production by muscle cells using plasmid DNA. J. Endocrinol. 2000;165:329–336. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

