In vivo imaging of hemodynamic redistribution and arteriogenesis across microvascular network
- PMID: 31660674
- PMCID: PMC7160018
- DOI: 10.1111/micc.12598
In vivo imaging of hemodynamic redistribution and arteriogenesis across microvascular network
Abstract
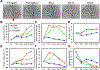
Objective: Arteriogenesis is an important mechanism that contributes to restoration of oxygen supply in chronically ischemic tissues, but remains incompletely understood due to technical limitations. This study presents a novel approach for comprehensive assessment of the remodeling pattern in a complex microvascular network containing multiple collateral microvessels.
Methods: We have developed a hardware-software integrated platform for quantitative, longitudinal, and label-free imaging of network-wide hemodynamic changes and arteriogenesis at the single-vessel level. By ligating feeding arteries in the mouse ear, we induced network-wide hemodynamic redistribution and localized arteriogenesis. The utility of this technology was demonstrated by studying the influence of obesity on microvascular arteriogenesis.
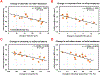
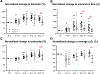
Results: Simultaneously monitoring the remodeling of competing collateral arterioles revealed a new, inverse relationship between initial vascular resistance and extent of arteriogenesis. Obese mice exhibited similar remodeling responses to lean mice through the first week, including diameter increase and flow upregulation in collateral arterioles. However, these gains were subsequently lost in obese mice.
Conclusions: Capable of label-free, comprehensive, and dynamic quantification of structural and functional changes in the microvascular network in vivo, this platform opens up new opportunities to study the mechanisms of microvascular arteriogenesis, its implications in diseases, and approaches to pharmacologically rectify microvascular dysfunction.
Keywords: arterial ligation; arteriogenesis; hemodynamic redistribution; microvascular remodeling; obesity.
© 2019 John Wiley & Sons Ltd.
Figures




References
-
- Brunner F, Tomandl B, Hanken K, Hildebrandt H, Kastrup A. Impact of collateral circulation on early outcome and risk of hemorrhagic complications after systemic thrombolysis. International Journal of Stroke. 2014;9(8):992–8. - PubMed
-
- Kimmel ER, Al Kasab S, Harvey JB, Bathla G, Ortega-Gutierrez S, Toth G, Jaksich EM, Sheharyar A, Roa J, Hasan DM, Samaniego EA. Absence of Collaterals is Associated with Larger Infarct Volume and Worse Outcome in Patients with Large Vessel Occlusion and Mild Symptoms. Journal of Stroke and Cerebrovascular Diseases. 2019;28(7):1987–1992. - PubMed
-
- Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(9):1664–1668. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

