Pain REduction with bone metastases STereotactic radiotherapy (PREST): A phase III randomized multicentric trial
- PMID: 31661034
- PMCID: PMC6816218
- DOI: 10.1186/s13063-019-3676-x
Pain REduction with bone metastases STereotactic radiotherapy (PREST): A phase III randomized multicentric trial
Abstract
Background: Palliative antalgic treatments represent an issue for clinical management and a challenge for scientific research. Radiotherapy (RT) plays a central role. Techniques such as stereotactic body radiotherapy (SBRT) were largely investigated in several phase 2 studies with good symptom response, becoming widely adopted. However, evidence from randomized, direct comparison of RT and SBRT is still lacking.
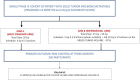
Methods/design: The PREST trial was designed as an interventional study without medicinal treatment. It is a phase 3, open-label, multicentric trial randomized 1:1. Inclusion criteria include painful spinal bone metastases presenting with a pain level > 4 (or > 1 if being treated with an analgesic) on the Numeric Rating Scale (NRS); expected intermediate/high prognosis (greater than 6 months) according to the Mizumoto prognostic score; low spine instability neoplastic score (SINS) sores (< 7); magnetic resonance imaging (MRI) assessment of the bulky lesion. Patients will be assigned to either standard conventional radiotherapy involving 4 Gy × 5 fractions (fx) to the whole involved vertebra or SBRT by intensity modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) involving 7 Gy × 3 fx to the whole involved vertebra + 10 Gy × 3 fx on the macroscopic lesion (gross tumor volume (GTV)). In the experimental arm, the GTV will be contoured by registration with baseline MRI.
Discussion: The primary endpoint is overall pain reduction, defined in terms of variation between baseline and 3-month evaluation; pain will be measured using the NRS. Secondary endpoints include pain control duration; retreatment rates (after a minimum interval of 1 month); local control assessed with RECIST criteria; symptom progression free survival; progression-free survival; overall survival; and quality of life (at 0, 30, and 90 days). Accrual of 330 lesions is planned. The experimental arm is expected to have an improvement in overall pain response rates of 15% with respect to the standard arm (60% according to Chow et al. (Int J Radiat Oncol Biol Phys. 82(5):1730-7, 2012)).
Trial registration: ClinicalTrials.gov, NCT03597984 . Registered on July 2018.
Keywords: Bone metastases; Pain control; Randomised controlled trial; Simultaneous integrated boost.
Conflict of interest statement
The authors declare that they have no competing interests.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical