Cerebellar climbing fibers encode expected reward size
- PMID: 31661073
- PMCID: PMC6844644
- DOI: 10.7554/eLife.46870
Cerebellar climbing fibers encode expected reward size
Abstract
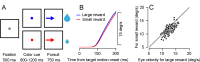
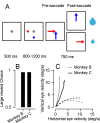
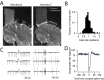
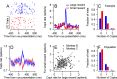
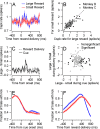
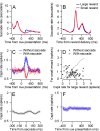
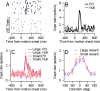
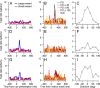
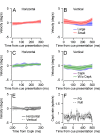
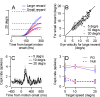
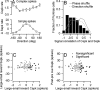
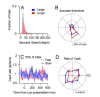
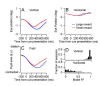
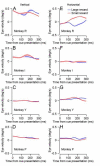
Climbing fiber inputs to the cerebellum encode error signals that instruct learning. Recently, evidence has accumulated to suggest that the cerebellum is also involved in the processing of reward. To study how rewarding events are encoded, we recorded the activity of climbing fibers when monkeys were engaged in an eye movement task. At the beginning of each trial, the monkeys were cued to the size of the reward that would be delivered upon successful completion of the trial. Climbing fiber activity increased when the monkeys were presented with a cue indicating a large reward, but not a small reward. Reward size did not modulate activity at reward delivery or during eye movements. Comparison between climbing fiber and simple spike activity indicated different interactions for coding of movement and reward. These results indicate that climbing fibers encode the expected reward size and suggest a general role of the cerebellum in associative learning beyond error correction.
Keywords: cerebellum; complex spikes; neuroscience; reinforcement learning; reward prediction; smooth pursuit.
© 2019, Larry et al.
Conflict of interest statement
NL, MY, AL, MJ No competing interests declared
Figures



References
-
- Albus JS. A theory of cerebellar function. Mathematical Biosciences. 1971;10:25–61. doi: 10.1016/0025-5564(71)90051-4. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

