Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension
- PMID: 31661308
- PMCID: PMC7047445
- DOI: 10.1164/rccm.201906-1141OC
Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension
Abstract
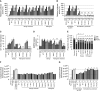
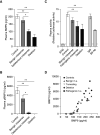
Rationale: Recently, rare heterozygous mutations in GDF2 were identified in patients with pulmonary arterial hypertension (PAH). GDF2 encodes the circulating BMP (bone morphogenetic protein) type 9, which is a ligand for the BMP2 receptor.Objectives: Here we determined the functional impact of GDF2 mutations and characterized plasma BMP9 and BMP10 levels in patients with idiopathic PAH.Methods: Missense BMP9 mutant proteins were expressed in vitro and the impact on BMP9 protein processing and secretion, endothelial signaling, and functional activity was assessed. Plasma BMP9 and BMP10 levels and activity were assayed in patients with PAH with GDF2 variants and in control subjects. Levels were also measured in a larger cohort of control subjects (n = 120) and patients with idiopathic PAH (n = 260).Measurements and Main Results: We identified a novel rare variation at the GDF2 and BMP10 loci, including copy number variation. In vitro, BMP9 missense proteins demonstrated impaired cellular processing and secretion. Patients with PAH who carried these mutations exhibited reduced plasma levels of BMP9 and reduced BMP activity. Unexpectedly, plasma BMP10 levels were also markedly reduced in these individuals. Although overall BMP9 and BMP10 levels did not differ between patients with PAH and control subjects, BMP10 levels were lower in PAH females. A subset of patients with PAH had markedly reduced plasma levels of BMP9 and BMP10 in the absence of GDF2 mutations.Conclusions: Our findings demonstrate that GDF2 mutations result in BMP9 loss of function and are likely causal. These mutations lead to reduced circulating levels of both BMP9 and BMP10. These findings support therapeutic strategies to enhance BMP9 or BMP10 signaling in PAH.
Keywords: BMP10; BMP9; GDF2; pulmonary arterial hypertension.
Figures


References
-
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med . 2004;351:1655–1665. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/15/59/31839/BHF_/British Heart Foundation/United Kingdom
- PG/12/54/29734/BHF_/British Heart Foundation/United Kingdom
- RG/08/006/25302/BHF_/British Heart Foundation/United Kingdom
- RG/19/3/34265/BHF_/British Heart Foundation/United Kingdom
- PG/17/1/32532/BHF_/British Heart Foundation/United Kingdom
- PG/17/58/33134/BHF_/British Heart Foundation/United Kingdom
- CH/09/001/25945/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- PG/15/39/31519/BHF_/British Heart Foundation/United Kingdom
- 204809/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/K020919/1/MRC_/Medical Research Council/United Kingdom
- RG/13/4/30107/BHF_/British Heart Foundation/United Kingdom
- FS/18/52/33808/BHF_/British Heart Foundation/United Kingdom
- FS/13/48/30453/BHF_/British Heart Foundation/United Kingdom
- FS/15/62/323032 /BHF_/British Heart Foundation/United Kingdom

