Population pharmacokinetics of vancomycin in obesity: Finding the optimal dose for (morbidly) obese individuals
- PMID: 31661553
- PMCID: PMC7015748
- DOI: 10.1111/bcp.14144
Population pharmacokinetics of vancomycin in obesity: Finding the optimal dose for (morbidly) obese individuals
Abstract
Aims: For vancomycin treatment in obese patients, there is no consensus on the optimal dose that will lead to the pharmacodynamic target (area under the curve 400-700 mg h L-1 ). This prospective study quantifies vancomycin pharmacokinetics in morbidly obese and nonobese individuals, in order to guide vancomycin dosing in the obese.
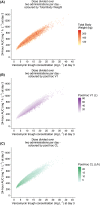
Methods: Morbidly obese individuals (n = 20) undergoing bariatric surgery and nonobese healthy volunteers (n = 8; total body weight [TBW] 60.0-234.6 kg) received a single vancomycin dose (obese: 12.5 mg kg-1 , maximum 2500 mg; nonobese: 1000 mg) with plasma concentrations measured over 48 h (11-13 samples per individual). Modelling, internal validation, external validation using previously published data and simulations (n = 10.000 individuals, TBW 60-230 kg) were performed using NONMEM.
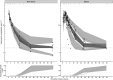
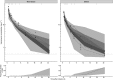
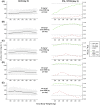
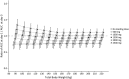
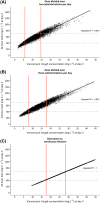
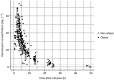
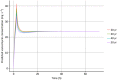
Results: In a 3-compartment model, peripheral volume of distribution and clearance increased with TBW (both p < 0.001), which was confirmed in the external validation. A dose of 35 mg kg-1 day-1 (maximum 5500 mg/day) resulted in a > 90% target attainment (area under the curve > 400 mg h L-1 ) in individuals up to 200 kg, with corresponding trough concentrations of 5.7-14.6 mg L-1 (twice daily dosing). For continuous infusion, a loading dose of 1500 mg is required for steady state on day 1.
Conclusion: In this prospective, rich sampling pharmacokinetic study, vancomycin clearance was well predicted using TBW. We recommend that in obese individuals without renal impairment, vancomycin should be dosed as 35 mg kg-1 day-1 (maximized at 5500 mg/day). When given over 2 daily doses, trough concentrations of 5.7-14.6 mg L-1 correspond to the target exposure in obese individuals.
Keywords: glycopeptides; morbid obesity; obesity; pharmacokinetics; pharmacology; vancomycin.
© 2019 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures


References
-
- Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438‐446. - PubMed
-
- Smit C, De Hoogd S, Brüggemann RJM, Knibbe CAJ. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275‐285. - PubMed
-
- Knibbe CAJ, Brill MJE, Van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence‐based dosing. Annu Rev Pharmacol Toxicol. 2015;55(1):149‐167. - PubMed
-
- Purrello SM, Garau J, Giamarellos E, et al. Methicillin‐resistant Staphylococcus aureus infections: a review of the currently available treatment options. J Glob Antimicrob Resist. 2016;7:178‐186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

