Fatty Acid-Binding Protein 3 is Critical for α-Synuclein Uptake and MPP+-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons
- PMID: 31661838
- PMCID: PMC6862506
- DOI: 10.3390/ijms20215358
Fatty Acid-Binding Protein 3 is Critical for α-Synuclein Uptake and MPP+-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons
Abstract
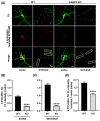
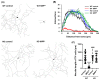
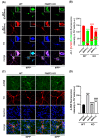
α-Synuclein is an abundant neuronal protein that accumulates in insoluble inclusions in Parkinson's disease and other synucleinopathies. Fatty acids partially regulate α-Synuclein accumulation, and mesencephalic dopaminergic neurons highly express fatty acid-binding protein 3 (FABP3). We previously demonstrated that FABP3 knockout mice show decreased α-Synuclein oligomerization and neuronal degeneration of tyrosine hydroxylase (TH)-positive neurons in vivo. In this study, we newly investigated the importance of FABP3 in α-Synuclein uptake, 1-methyl-4-phenylpyridinium (MPP+)-induced axodendritic retraction, and mitochondrial dysfunction. To disclose the issues, we employed cultured mesencephalic neurons derived from wild type or FABP3-/- C57BL6 mice and performed immunocytochemical analysis. We demonstrated that TH+ neurons from FABP3+/+ mice take up α-Synuclein monomers while FABP3-/- TH+ neurons do not. The formation of filamentous α-Synuclein inclusions following treatment with MPP+ was observed only in FABP3+/+, and not in FABP3-/- neurons. Notably, detailed morphological analysis revealed that FABP-/- neurons did not exhibit MPP+-induced axodendritic retraction. Moreover, FABP3 was also critical for MPP+-induced reduction of mitochondrial activity and the production of reactive oxygen species. These data indicate that FABP3 is critical for α-Synuclein uptake in dopaminergic neurons, thereby preventing synucleinopathies, including Parkinson's disease.
Keywords: 1-methyl-4-phenylpyridinium (MPP+); Parkinson’s disease; fatty acid-binding protein 3; mitochondria; synucleinopathy; α-Synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

