Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan
- PMID: 31661916
- PMCID: PMC6895815
- DOI: 10.3390/genes10110853
Comparative Physiological and Transcriptomic Analyses Reveal Mechanisms of Improved Osmotic Stress Tolerance in Annual Ryegrass by Exogenous Chitosan
Abstract
Water deficit adversely affects the growth and productivity of annual ryegrass (Lolium multiflorum Lam.). The exogenous application of chitosan (CTS) has gained extensive interests due to its effect on improving drought resistance. This research aimed to determine the role of exogenous CTS on annual ryegrass in response to water stress. Here, we investigated the impact of exogenous CTS on the physiological responses and transcriptome changes of annual ryegrass variety "Tetragold" under osmotic stress induced by exposing them to 20% polyethylene glycol (PEG)-6000. Our experimental results demonstrated that 50 mg/L exogenous CTS had the optimal effect on promoting seed germination under osmotic stress. Pre-treatment of annual ryegrass seedlings with 500 mg/L CTS solution reduced the level of electrolyte leakage (EL) as well as the contents of malondialdehyde (MDA) and proline and enhanced the activities of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbic acid peroxidase (APX) under osmotic stress. In addition, CTS increased soluble sugars and chlorophyll (Chl) content, net photosynthetic rate (A), stomatal conductance (gs), water use efficiency (WUE), and transpiration rate (E) in annual ryegrass seedlings in response to three and six days of osmotic stress. Transcriptome analysis further provided a comprehensive understanding of underlying molecular mechanisms of CTS impact. To be more specific, in contrast of non-treated seedlings, the distinct changes of gene expressions of CTS-treated seedlings were shown to be tightly related to carbon metabolism, photosynthesis, and plant hormone. Altogether, exogenous CTS could elicit drought-related genes in annual ryegrass, leading to resistance to osmotic stress via producing antioxidant enzymes and maintaining intact cell membranes and photosynthetic rates. This robust evidence supports the potential of the application of exogenous CTS, which will be helpful for determining the suitability and productivity of agricultural crops.
Keywords: Lolium multiflorum Lam.; antioxidant enzymes; exogenous chitosan; osmotic stress; physiological and photosynthetic characterizes; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





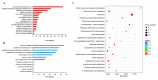


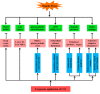
References
-
- Pongprayoon W., Roytrakul S., Pichayangkura R., Chadchawan S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa l.) Plant Growth Regul. 2013;70:159–173. doi: 10.1007/s10725-013-9789-4. - DOI
-
- Bittelli M., Flury M., Campbell G.S., Nichols E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001;107:167–175. doi: 10.1016/S0168-1923(00)00242-2. - DOI
-
- Yang F., Hu J.J., Li J.L., Wu X.L., Qian Y.R. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Growth Regul. 2009;58:131–136. doi: 10.1007/s10725-009-9361-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

