Experimental Design Modeling of the Effect of Hexagonal Wurtzite-ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent
- PMID: 31661919
- PMCID: PMC6864852
- DOI: 10.3390/molecules24213884
Experimental Design Modeling of the Effect of Hexagonal Wurtzite-ZnO Synthesis Conditions on Its Characteristics and Performance as a Cationic and Anionic Adsorbent
Abstract
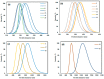
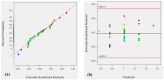
Surface composite design was used to study the effect of the ZnO synthesis conditions on its adsorption of methyl orange (MO) and methylene blue (MB). The ZnO was prepared via hydrothermal treatment under different conditions including temperature (T), precursor concentration (C), pH, and reaction time (t). Models were built using four Design expert-11 software-based responses: the point of zero charge (pHzc), MO and MB removal efficiencies (RMO, RMB), MO and MB adsorption capacities (qMO, qMB), and hydrodynamic diameter of ZnO particles (Dh). ZnO was characterized by X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, UV/VIS spectroscopy, thermal gravimetric analysis (TGA), and dynamic light scattering (DLS). The formation of ZnO was confirmed by the XRD, UV, and FTIR spectra. Results showed a very high efficiency for most of the samples for adsorption of MB, and more than 90% removal efficiency was achieved by 8 samples among 33 samples. For MO, more than 90% removal efficiency was achieved by 2 samples among 33 samples. Overall, 26 of 31 samples showed higher MB adsorption capacity than that of MO. RMB was found to depend only on the synthesis temperature while RMO depends on temperature, pH, and reaction time. pHzc was found to be affected by the synthesis pH only while Dh depends on the synthesis pH and precursor concentration.
Keywords: ZnO; adsorption; methyl orange; methylene blue; nanoparticles; surface composite design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Duo S., Li Y., Liu Z., Zhong R., Liu T. Novel hybrid self-assembly of an ultralarge ZnO macroflower and defect intensity-induced photocurrent and photocatalytic properties by facile hydrothermal synthesis using CO(NH2)2–N2H4 as alkali sources. Mater. Sci. Semicond. Process. 2016;56:196–212. doi: 10.1016/j.mssp.2016.08.018. - DOI
-
- Andrade G.R., Nascimento C.C., Lima Z.M., Costa L.P., Gimenez I.F., Teixeira-Neto E. Star-shaped ZnO/Ag hybrid nanostructures for enhanced photocatalysis and antibacterial activity. Appl. Surf. Sci. 2017;399:573–582. doi: 10.1016/j.apsusc.2016.11.202. - DOI
-
- Fiedot M., Maliszewska I., Rac-Rumijowska O., Suchorska-Woźniak P., Lewińska A., Teterycz H. The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes. Materials. 2017;10:353. doi: 10.3390/ma10040353. - DOI - PMC - PubMed
-
- Ali T.T., Narasimharao K., Parkin I.P., Carmalt C.J., Sathasivam S., Basahel S.N., Bawaked S.M., Al-Thabaiti S.A. Effect of pretreatment temperature on the photocatalytic activity of microwave irradiated porous nanocrystalline ZnO. New J. Chem. 2015;39:321–332. doi: 10.1039/C4NJ01465K. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

