Ascorbate Deficiency Does Not Limit Nonphotochemical Quenching in Chlamydomonas reinhardtii
- PMID: 31662419
- PMCID: PMC6945847
- DOI: 10.1104/pp.19.00916
Ascorbate Deficiency Does Not Limit Nonphotochemical Quenching in Chlamydomonas reinhardtii
Abstract
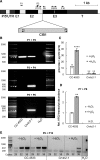
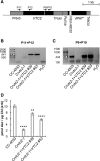
Ascorbate (Asc; vitamin C) plays essential roles in development, signaling, hormone biosynthesis, regulation of gene expression, stress resistance, and photoprotection. In vascular plants, violaxanthin de-epoxidase requires Asc as a reductant; thereby, Asc is required for the energy-dependent component of nonphotochemical quenching (NPQ). To assess the role of Asc in NPQ in green algae, which are known to contain low amounts of Asc, we searched for an insertional Chlamydomonas reinhardtii mutant affected in theVTC2 gene encoding GDP-l-Gal phosphorylase, which catalyzes the first committed step in the biosynthesis of Asc. The Crvtc2-1 knockout mutant was viable and, depending on the growth conditions, contained 10% to 20% Asc relative to its wild type. When C. reinhardtii was grown photomixotrophically at moderate light, the zeaxanthin-dependent component of NPQ emerged upon strong red illumination both in the Crvtc2-1 mutant and in its wild type. Deepoxidation was unaffected by Asc deficiency, demonstrating that the Chlorophycean violaxanthin de-epoxidase found in C. reinhardtii does not require Asc as a reductant. The rapidly induced, energy-dependent NPQ component characteristic of photoautotrophic C. reinhardtii cultures grown at high light was not limited by Asc deficiency either. On the other hand, a reactive oxygen species-induced photoinhibitory NPQ component was greatly enhanced upon Asc deficiency, both under photomixotrophic and photoautotrophic conditions. These results demonstrate that Asc has distinct roles in NPQ formation in C. reinhardtii as compared to vascular plants.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures






Similar articles
-
Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo.Plant Physiol. 2002 Mar;128(3):970-7. doi: 10.1104/pp.010924. Plant Physiol. 2002. PMID: 11891252 Free PMC article.
-
Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the VTC2 gene encoding GDP-l-galactose phosphorylase.New Phytol. 2017 Apr;214(2):668-681. doi: 10.1111/nph.14425. Epub 2017 Jan 23. New Phytol. 2017. PMID: 28112386
-
Chlamydomonas reinhardtii Exhibits De Facto Constitutive NPQ Capacity in Physiologically Relevant Conditions.Plant Physiol. 2020 Jan;182(1):472-479. doi: 10.1104/pp.19.00658. Epub 2019 Oct 25. Plant Physiol. 2020. PMID: 31653716 Free PMC article.
-
The physiological roles and metabolism of ascorbate in chloroplasts.Physiol Plant. 2013 Jun;148(2):161-75. doi: 10.1111/ppl.12006. Epub 2012 Dec 6. Physiol Plant. 2013. PMID: 23163968 Review.
-
Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants.Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1385-94. doi: 10.1098/rstb.2000.0700. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11127993 Free PMC article. Review.
Cited by
-
Methyl Jasmonate and Methyl-β-Cyclodextrin Individually Boost Triterpenoid Biosynthesis in Chlamydomonas Reinhardtii UVM4.Pharmaceuticals (Basel). 2021 Feb 5;14(2):125. doi: 10.3390/ph14020125. Pharmaceuticals (Basel). 2021. PMID: 33562714 Free PMC article.
-
Regulation of Microalgal Photosynthetic Electron Transfer.Plants (Basel). 2024 Jul 29;13(15):2103. doi: 10.3390/plants13152103. Plants (Basel). 2024. PMID: 39124221 Free PMC article. Review.
-
Insights into the binding mechanism of ascorbic acid and violaxanthin with violaxanthin de-epoxidase (VDE) and chlorophycean violaxanthin de-epoxidase (CVDE) enzymes.Photosynth Res. 2023 Jun;156(3):337-354. doi: 10.1007/s11120-023-01006-0. Epub 2023 Feb 27. Photosynth Res. 2023. PMID: 36847893
-
The ascorbate biosynthesis pathway in plants is known, but there is a way to go with understanding control and functions.J Exp Bot. 2024 May 3;75(9):2604-2630. doi: 10.1093/jxb/erad505. J Exp Bot. 2024. PMID: 38300237 Free PMC article. Review.
-
Chlamydomonas as a model for reactive oxygen species signaling and thiol redox regulation in the green lineage.Plant Physiol. 2021 Oct 5;187(2):687-698. doi: 10.1093/plphys/kiab355. Plant Physiol. 2021. PMID: 35237823 Free PMC article. Review.
References
-
- Adams WW III, Muller O, Cohu CM, Demmig-Adams B (2013) May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth Res 117: 31–44 - PubMed
-
- Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283: 3550–3558 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

