The ribosome assembly factor Nop53 controls association of the RNA exosome with pre-60S particles in yeast
- PMID: 31662437
- PMCID: PMC6916480
- DOI: 10.1074/jbc.RA119.010193
The ribosome assembly factor Nop53 controls association of the RNA exosome with pre-60S particles in yeast
Abstract
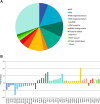
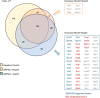
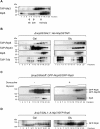
Eukaryotic ribosomal biogenesis is a high-energy-demanding and complex process that requires hundreds of trans-acting factors to dynamically build the highly-organized 40S and 60S subunits. Each ribonucleoprotein complex comprises specific rRNAs and ribosomal proteins that are organized into functional domains. The RNA exosome complex plays a crucial role as one of the pre-60S-processing factors, because it is the RNase responsible for processing the 7S pre-rRNA to the mature 5.8S rRNA. The yeast pre-60S assembly factor Nop53 has previously been shown to associate with the nucleoplasmic pre-60S in a region containing the "foot" structure assembled around the 3' end of the 7S pre-rRNA. Nop53 interacts with 25S rRNA and with several 60S assembly factors, including the RNA exosome, specifically, with its catalytic subunit Rrp6 and with the exosome-associated RNA helicase Mtr4. Nop53 is therefore considered the adaptor responsible for recruiting the exosome complex for 7S processing. Here, using proteomics-based approaches in budding yeast to analyze the effects of Nop53 on the exosome interactome, we found that the exosome binds pre-ribosomal complexes early during the ribosome maturation pathway. We also identified interactions through which Nop53 modulates exosome activity in the context of 60S maturation and provide evidence that in addition to recruiting the exosome, Nop53 may also be important for positioning the exosome during 7S processing. On the basis of these findings, we propose that the exosome is recruited much earlier during ribosome assembly than previously thought, suggesting the existence of additional interactions that remain to be described.
Keywords: exosome complex; pre-60S particles; precursor ribosomal RNA (pre-rRNA); protein–protein interaction; rRNA processing; ribosome assembly.
© 2019 Cepeda et al.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

