Shielding the Next Generation: Symbiotic Bacteria from a Reproductive Organ Protect Bobtail Squid Eggs from Fungal Fouling
- PMID: 31662458
- PMCID: PMC6819662
- DOI: 10.1128/mBio.02376-19
Shielding the Next Generation: Symbiotic Bacteria from a Reproductive Organ Protect Bobtail Squid Eggs from Fungal Fouling
Abstract
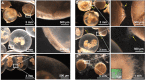
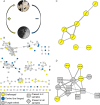
The importance of defensive symbioses, whereby microbes protect hosts through the production of specific compounds, is becoming increasingly evident. Although defining the partners in these associations has become easier, assigning function to these relationships often presents a significant challenge. Here, we describe a functional role for a bacterial consortium in a female reproductive organ in the Hawaiian bobtail squid, Euprymna scolopes Bacteria from the accessory nidamental gland (ANG) are deposited into the egg jelly coat (JC), where they are hypothesized to play a defensive role during embryogenesis. Eggs treated with an antibiotic cocktail developed a microbial biomass primarily composed of the pathogenic fungus Fusarium keratoplasticum that infiltrated the JC, resulting in severely reduced hatch rates. Experimental manipulation of the eggs demonstrated that the JC was protective against this fungal fouling. A large proportion of the bacterial strains isolated from the ANG or JC inhibited F. keratoplasticum in culture (87.5%), while a similar proportion of extracts from these strains also exhibited antifungal activity against F. keratoplasticum and/or the human-pathogenic yeast Candida albicans (72.7%). Mass spectral network analyses of active extracts from bacterial isolates and egg clutches revealed compounds that may be involved in preventing microbial overgrowth. Several secondary metabolites were identified from ANG/JC bacteria and egg clutches, including the known antimicrobial lincomycin as well as a suite of glycerophosphocholines and mycinamicin-like compounds. These results shed light on a widely distributed but poorly understood symbiosis in cephalopods and offer a new source for exploring bacterial secondary metabolites with antimicrobial activity.IMPORTANCE Organisms must have strategies to ensure successful reproduction. Some animals that deposit eggs protect their embryos from fouling/disease with the help of microorganisms. Although beneficial bacteria are hypothesized to contribute to egg defense in some organisms, the mechanisms of this protection remain largely unknown, with the exception of a few recently described systems. Using both experimental and analytical approaches, we demonstrate that symbiotic bacteria associated with a cephalopod reproductive gland and eggs inhibit fungi. Chemical analyses suggest that these bacteria produce antimicrobial compounds that may prevent overgrowth from fungi and other microorganisms. Given the distribution of these symbiotic glands among many cephalopods, similar defensive relationships may be more common in aquatic environments than previously realized. Such defensive symbioses may also be a rich source for the discovery of new antimicrobial compounds.
Keywords: Euprymna scolopes; chemical defense; defensive symbiosis; host-microbe symbioses; interaction-driven molecular networking.
Copyright © 2019 Kerwin et al.
Figures




References
-
- Lopanik N. 2014. Chemical defensive symbioses in the marine environment. Funct Ecol 28:328–340. doi: 10.1111/1365-2435.12160. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
