Healing of Bone Defects in Pig's Femur Using Mesenchymal Cells Originated from the Sinus Membrane with Different Scaffolds
- PMID: 31662765
- PMCID: PMC6791246
- DOI: 10.1155/2019/4185942
Healing of Bone Defects in Pig's Femur Using Mesenchymal Cells Originated from the Sinus Membrane with Different Scaffolds
Abstract
Objective: Repairing bone defects, especially in older individuals with limited regenerative capacity, is still a big challenge. The use of biomimetic materials that can enhance the restoration of bone structure represents a promising clinical approach. In this study, we evaluated ectopic bone formation after the transplantation of human maxillary Schneiderian sinus membrane- (hMSSM-) derived cells embedded within various scaffolds in the femur of pigs.
Methods: The scaffolds used were collagen, gelatin, and hydroxyapatite/tricalcium phosphate (HA/βTCP) where fibrin/thrombin was used as a control. Histological analysis was performed for the new bone formation. Quantitative real-time PCR (qRT-PCR) and immunohistochemistry (IHC) were used to assess mRNA and protein levels of specific osteoblastic markers, respectively.
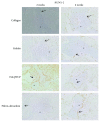
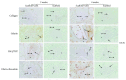
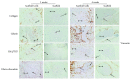
Results: Histological analysis showed that the three scaffolds we used can support new bone formation with a more pronounced effect observed in the case of the gelatin scaffold. In addition, mRNA levels of the different tested osteoblastic markers Runt-Related Transcription Factor 2 (RUNX-2), osteonectin (ON), osteocalcin (OCN), osteopontin (OPN), alkaline phosphatase (ALP), and type 1 collagen (COL1) were higher, after 2 and 4 weeks, in cell-embedded scaffolds than in control cells seeded within the fibrin/thrombin scaffold. Moreover, there was a very clear and differential expression of RUNX-2, OCN, and vimentin in osteocytes, osteoblasts, hMSSM-derived cells, and bone matrix. Interestingly, the osteogenic markers were more abundant, at both time points, in cell-embedded gelatin scaffold than in other scaffolds (collagen, HA/βTCP, fibrin/thrombin).
Conclusions: These results hold promise for the development of successful bone regeneration techniques using different scaffolds embedded with hMSSM-derived cells. This trial is registered with NCT02676921.
Copyright © 2019 Rita Bou Assaf et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures




References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

