GC-MS Analysis and Inhibitory Evaluation of Terminalia catappa Leaf Extracts on Major Enzymes Linked to Diabetes
- PMID: 31662777
- PMCID: PMC6748200
- DOI: 10.1155/2019/6316231
GC-MS Analysis and Inhibitory Evaluation of Terminalia catappa Leaf Extracts on Major Enzymes Linked to Diabetes
Abstract
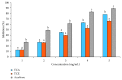
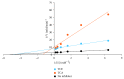
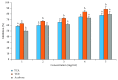
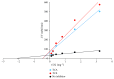
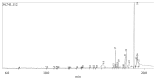
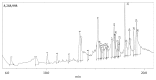
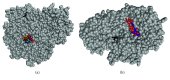
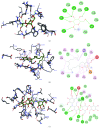
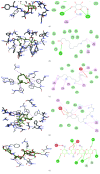
Terminalia catappa leaves are used in managing both diabetes mellitus and its complications in Southwest Nigeria. However, its inhibitory activity on enzymes implicated in diabetes is not very clear. This study investigated the in vitro inhibitory properties and mode of inhibition of T. catappa leaf extracts on enzymes associated with diabetes. The study also identified some bioactive compounds as well as their molecular interaction in the binding pocket of these enzymes. Standard enzyme inhibition and kinetics assays were performed to determine the inhibitory effects of aqueous extract (TCA) and ethanol extract (TCE) of T. catappa leaves on α-glucosidase and α-amylase activities. The phytoconstituents of TCA and TCE were determined using GC-MS. Molecular docking of the phytocompounds was performed using Autodock Vina. TCA and TCE were the most potent inhibitors of α-glucosidase (IC50 = 3.28 ± 0.47 mg/mL) and α-amylase (IC50 = 0.24 ± 0.08 mg/mL), respectively. Both extracts displayed a mixed mode of inhibition on α-amylase activity, while mixed and noncompetitive modes of inhibition were demonstrated by TCA and TCE, respectively, on α-glucosidase activity. The GC-MS analytic chromatogram revealed the presence of 24 and 22 compounds in TCE and TCA, respectively, which were identified mainly as phenolic compounds, terpenes/terpenoids, fatty acids, and other phytochemicals. The selected compounds exhibited favourable interactions with the enzymes compared with acarbose. Overall, the inhibitory effect of T. catappa on α-amylase and α-glucosidase may be ascribed to the synergistic action of its rich phenolic and terpene composition giving credence to the hypoglycaemic nature of T. catappa leaves.
Copyright © 2019 Franklyn Nonso Iheagwam et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures
References
-
- International Diabetes Federation. IDF Diabetes Atlas. 8th. Brussels, Belgium: International Diabetes Federation; 2017. http://www.diabetesatlas.org. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

