A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma
- PMID: 31662965
- PMCID: PMC6778921
- DOI: 10.1155/2019/1092563
A Preliminary Study of Biliary Microbiota in Patients with Bile Duct Stones or Distal Cholangiocarcinoma
Abstract
Background and objective: The distal cholangiocarcinoma (dCCA) is associated with many factors: genes, environment, infection, etc. The current changes in biliary flora are thought to be involved in the formation of many gastrointestinal tract (GIT) diseases, like colon adenocarcinoma. Therefore we want to investigate whether the dCCA has a certain correlation with biliary microecology, and to detect specific strains.
Methods: A total of 68 adults were enrolled, of whom 8 with dCCA, 16 with recurrent choledocholithiasis, and 44 with the onset of common bile duct stones. Endoscopic Retrograde Cholangiopancretography (ERCP) was utilized to collect bile samples for DNA extraction and 16S rRNA gene sequencing, followed by analysis of bile microbiota composition.
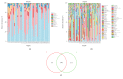
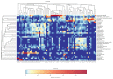
Results: First, Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria are the most dominant phyla in the bile of patients with dCCA and the onset of common bile duct stoes. Secondly, compared with the onset of common bile duct stones patients, we got a significant increase in the phylum Gemmatimonadetes, Nitrospirae, Chloroflexi, Latescibacteria, and Planctomycetes in dCCA patients. Finally, at the genus level, we obtained sequencing results of 252 bacterial genera from patients with dCCA, recurrent choledocholithiasis, and the new onset of common bile duct stones, revealing heterogeneity among individuals.
Conclusion: To the best of our knowledge, this is the first study of the dysbiosis of bile flora in patients with dCCA. This micro-ecological disorder may be a decisive factor in the formation of dCCA. At the same time, for the first time, this study provides a test chart of biliary microbial populations that may be associated with recurrent choledocholithiasis. The compositional changes of the core microbial group of the biliary tract have potentially important biological and medical significance for the microbiological biliary disorders of dCCA.
Copyright © 2019 Bingrong Chen et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

