Effectiveness of medication review on the number of drug-related problems in patients visiting the outpatient cardiology clinic: A randomized controlled trial
- PMID: 31663156
- PMCID: PMC6983519
- DOI: 10.1111/bcp.14125
Effectiveness of medication review on the number of drug-related problems in patients visiting the outpatient cardiology clinic: A randomized controlled trial
Abstract
Aims: To assess the effectiveness of medication review on the number of drug-related problems (DRPs) in outpatient cardiology patients.
Methods: In this randomized controlled trial, a computer-assisted and pharmacist-led medication review with patient involvement (questionnaire and telephone call with pharmacist) was conducted in intervention patients prior to their visit to the cardiologist. The control group received usual care. Adult outpatient cardiology patients without support concerning the administration of medication, without a medication review in the past 6 months and who gave permission to access their electronic medication record were included. The primary outcome measure was the number of DRPs 1 month after the visit. Secondary outcome measures concerned the type of DRP and the type of medication involved in the DRPs.
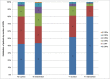
Results: In total, 75 patients (mean [standard deviation, SD] age 66.0 [12.5] years, 41% female) were included. Intervention (n = 90) and control group (n = 85) were comparable at baseline. The mean (SD) number of drugs used per patient was 7.9 (3.9). After 1 month, the mean (SD) number of DRPs was 0.3 (0.7) and 0.8 (1.0) and the median (range) number of DRPs was 0 (0-4) and 0 (0-4) in the intervention group and control group, respectively (P < .001). In the intervention group, 74% of the DRPs identified at T0 were solved at T1 vs 14% in the control group.
Conclusion: This randomized controlled trial suggests that a pharmacist-led medication review in patients with a scheduled visit to the outpatient cardiology clinic decreases the number of DRPs.
Keywords: clinical pharmacology; drug development; drug safety; drug utilization; prescribing; quality use of medicines; randomised controlled trial.
© 2019 The British Pharmacological Society.
Conflict of interest statement
There are no competing interests to declare.
Figures


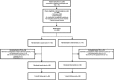
= live consult between cardiologist and patient (usual care). Furthermore, only in the intervention group, in addition to the usual care, communication between the cardiologist and the patient (live during consult) about the implementation of solutions to DRPs.

= patient questionnaire to make an inventory of the patient's experiences with the use of their medicines.

= medication review form, which is used by the pharmacist to perform the medication review. The items that were to be assessed during the medication review were shown on the medication review form. Assessment of all these items results in potential DRPs.

= telephone call between pharmacist and patient to assess which potential DRPs that were found during the medication review at t = 0 (baseline) are actual/real DRPs according to the patient, and to assess which actual/real DRPs were solved at t = 1 (1 month after the consult).

= communication form between the pharmacist and cardiologist about DRPs. The pharmacist reported to the cardiologist which potential DRPs were found during the medication review at t = 0 (baseline). The cardiologist judged whether these potential DRPs were actual/real DRPs
References
-
- Pharmaceutical Care Network Europe Foundation . The PCNE classification V 6.2. https://www.pcne.org/upload/files/11_PCNE_classification_V6-2.pdf.
-
- van den Bemt PM, Egberts TC, de Jong‐van den Berg LT, Brouwers JR. Drug‐related problems in hospitalised patients. Drug Saf. 2000;22(4):321‐333. - PubMed
-
- Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890‐1896. - PubMed
-
- Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug‐related hospital admissions. Ann Pharmacother. 2002;36(7‐8):1238‐1248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

