Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer's disease models
- PMID: 31663252
- PMCID: PMC6974721
- DOI: 10.1111/acel.13054
Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer's disease models
Abstract
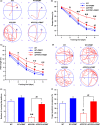
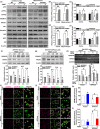
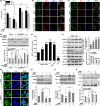
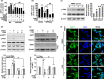
A hallmark of Alzheimer's disease (AD) is the accumulation of amyloid-β (Aβ), which correlates significantly with progressive cognitive deficits. Although photobiomodulation therapy (PBMT), as a novel noninvasive physiotherapy strategy, has been proposed to improve neuronal survival, decrease neuron loss, ameliorate dendritic atrophy, and provide overall AD improvement, it remains unknown whether and how this neuroprotective process affects Aβ levels. Here, we report that PBMT reduced Aβ production and plaque formation by shifting amyloid precursor protein (APP) processing toward the nonamyloidogenic pathway, thereby improving memory and cognitive ability in a mouse model of AD. More importantly, a pivotal protein, SIRT1, was involved in this process by specifically up-regulating ADAM10 and down-regulating BACE1, which is dependent on the cAMP/PKA pathway in APP/PS1 primary neurons and SH-SY5Y cells stably expressing human APP Swedish mutation (APPswe). We further found that the activity of the mitochondrial photoacceptor cytochrome c oxidase (CcO) was responsible for PBMT-induced activation of PKA and SIRT1. Together, our research suggests that PBMT as a viable therapeutic strategy has great potential value in improving cognitive ability and combatting AD.
Keywords: APP processing; Alzheimer's disease; SIRT1; amyloid-β; cAMP/PKA pathway; photobiomodulation therapy.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Billings, L. M. , Oddo, S. , Green, K. N. , McGaugh, J. L. , & LaFerla, F. M. (2005). Intraneuronal Abeta causes the onset of early Alzheimer's disease‐related cognitive deficits in transgenic mice. Neuron, 45, 675–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

