Identification and management of pancreas divisum
- PMID: 31663403
- PMCID: PMC6872911
- DOI: 10.1080/17474124.2019.1685871
Identification and management of pancreas divisum
Abstract
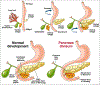
Introduction: Pancreas divisum is the most common congenital malformation of the pancreas with the majority asymptomatic. The etiological role, pathogenesis, clinical significance and management of pancreas divisum in pancreatic disease has not been clearly defined and our understanding is yet to be fully elucidated.Areas covered: This review describes the role of pancreas divisum in the development of pancreatic disease and the ambiguity related to it. In our attempt to offer clarity, a comprehensive search on PubMed, Ovid, Embase and Cochrane Library from inception to May 2019 was undertaken using key words "pancreas divisum", "idiopathic recurrent acute pancreatitis" and "chronic pancreatitis".Expert opinion: Current research fails to define a clear association between pancreas divisum and pancreatic disease. Though debatable, several studies do suggest a pathological role of pancreas divisum in pancreatic disease and a benefit of minor papilla therapy in the setting of acute recurrent pancreatitis. Surgical and endoscopic therapeutic modalities have not been directly compared. With the current data available, it would be imprudent to advise a definitive line of management for pancreatic disease associated with pancreas divisum and should involve a comprehensive discussion with the individual patient to define expectations before embarking on any medical and/or interventional therapy.
Keywords: Recurrent acute pancreatitis; chronic pancreatitis; dorsal duct syndrome; endoscopic retrograde cholangiopancreatography; idiopathic pancreatitis; magnetic resonance cholangiopancreatography; pancreas divisum.
Figures










References
-
- Kozu T, Suda K, Toki F. Pancreatic development and anatomical variation. Gastrointestinal endoscopy clinics of North America. 1995. January;5(1):1–30. - PubMed
-
- Smanio T Proposed nomenclature and classification of the human pancreatic ducts and duodenal papillae. Study based on 200 post mortems. International surgery. 1969. August;52(2):125–41. - PubMed
-
- Stimec B, Bulajic M, Korneti V, et al. Ductal morphometry of ventral pancreas in pancreas divisum. Comparison between clinical and anatomical results. The Italian journal of gastroenterology. 1996. Feb-Mar;28(2):76–80. - PubMed
-
- Bernard JP, Sahel J, Giovannini M, et al. Pancreas divisum is a probable cause of acute pancreatitis: a report of 137 cases. Pancreas. 1990. May;5(3):248–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
