Kv2.1 mediates spatial and functional coupling of L-type calcium channels and ryanodine receptors in mammalian neurons
- PMID: 31663850
- PMCID: PMC6839919
- DOI: 10.7554/eLife.49953
Kv2.1 mediates spatial and functional coupling of L-type calcium channels and ryanodine receptors in mammalian neurons
Abstract
The voltage-gated K+ channel Kv2.1 serves a major structural role in the soma and proximal dendrites of mammalian brain neurons, tethering the plasma membrane (PM) to endoplasmic reticulum (ER). Although Kv2.1 clustering at neuronal ER-PM junctions (EPJs) is tightly regulated and highly conserved, its function remains unclear. By identifying and evaluating proteins in close spatial proximity to Kv2.1-containing EPJs, we discovered that a significant role of Kv2.1 at EPJs is to promote the clustering and functional coupling of PM L-type Ca2+ channels (LTCCs) to ryanodine receptor (RyR) ER Ca2+ release channels. Kv2.1 clustering also unexpectedly enhanced LTCC opening at polarized membrane potentials. This enabled Kv2.1-LTCC-RyR triads to generate localized Ca2+ release events (i.e., Ca2+ sparks) independently of action potentials. Together, these findings uncover a novel mode of LTCC regulation and establish a unique mechanism whereby Kv2.1-associated EPJs provide a molecular platform for localized somatodendritic Ca2+ signals in mammalian brain neurons.
Keywords: calcium signaling; ion channel; membrane contact sites; molecular biophysics; mouse; neuroscience; rat; structural biology.
© 2019, Vierra et al.
Conflict of interest statement
NV, MK, Dv, LS, JT No competing interests declared
Figures


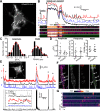
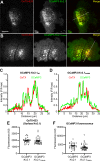
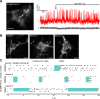
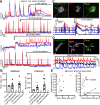
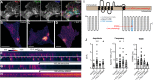


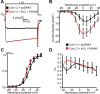
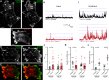
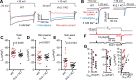

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

