Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone
- PMID: 31664021
- PMCID: PMC6820566
- DOI: 10.1038/s41467-019-12895-y
Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone
Abstract
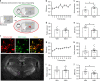
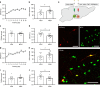
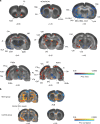
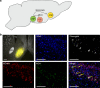
Behavioral impulsivity is common in various psychiatric and metabolic disorders. Here we identify a hypothalamus to telencephalon neural pathway for regulating impulsivity involving communication from melanin-concentrating hormone (MCH)-expressing lateral hypothalamic neurons to the ventral hippocampus subregion (vHP). Results show that both site-specific upregulation (pharmacological or chemogenetic) and chronic downregulation (RNA interference) of MCH communication to the vHP increases impulsive responding in rats, indicating that perturbing this system in either direction elevates impulsivity. Furthermore, these effects are not secondary to either impaired timing accuracy, altered activity, or increased food motivation, consistent with a specific role for vHP MCH signaling in the regulation of impulse control. Results from additional functional connectivity and neural pathway tracing analyses implicate the nucleus accumbens as a putative downstream target of vHP MCH1 receptor-expressing neurons. Collectively, these data reveal a specific neural circuit that regulates impulsivity and provide evidence of a novel function for MCH on behavior.
Conflict of interest statement
M.R.H. has received research support from investigator-initiated sponsored proposals from Novo Nordisk, Zealand Pharma, and Boehringer-Ingelheim. The other authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

