CD47 Blocking Antibody Accelerates Hematoma Clearance After Intracerebral Hemorrhage in Aged Rats
- PMID: 31664629
- PMCID: PMC7190411
- DOI: 10.1007/s12975-019-00745-4
CD47 Blocking Antibody Accelerates Hematoma Clearance After Intracerebral Hemorrhage in Aged Rats
Abstract
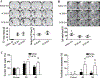
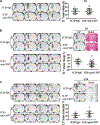
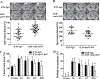
Both experimental studies and surgical clinical trials suggest that hematoma clearance is a therapeutic target in intracerebral hemorrhage (ICH). We have investigated effects of CD47, a "don't eat me" signal expressed on erythrocytes, on hematoma resolution after ICH in young mice. This study expands those findings by examining the effects on a CD47 blocking antibody in aged rats. First, male Fischer 344 rats (18 months old) received an intracaudate injection of 50 μL autologous whole blood or saline. Hematoma features of magnetic resonance imaging (MRI) and neurological deficits were evaluated within 3 days. Second, rats had an intracaudate co-injection of 50 μL autologous blood with either CD47 blocking antibody or IgG. MRI was used to quantify hematoma/iron volume, hemolysis, brain swelling, and atrophy at different time points, behavioral tests to assess neurological deficits, and immunohistochemistry to assess brain injury and neuroinflammation. The CD47 blocking antibody significantly promoted hematoma clearance, attenuated brain swelling, hemolysis, and neuronal loss and increased the number of phagocytic macrophages in and around hematoma 3 days after ICH. Moreover, CD47 blockade reduced neuronal loss, brain atrophy, and neurobehavioral deficits at day 28. These results indicate that a CD47 blocking antibody can accelerate hematoma clearance and alleviate short- and long-term brain injury after ICH in aged rats and that it might be a therapeutic strategy for ICH.
Keywords: Aged rats; CD47 blocking antibody; Cerebral hemorrhage; Macrophage activation.
Conflict of interest statement
Compliance with Ethical Standards
Figures

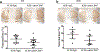

References
-
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993 - PubMed
-
- Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (mistie iii): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393:1021–1032 - PMC - PubMed
-
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: Role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

