Monoclonal antibody-based capture ELISA in the diagnosis of previous dengue infection
- PMID: 31665014
- PMCID: PMC6820907
- DOI: 10.1186/s12985-019-1222-9
Monoclonal antibody-based capture ELISA in the diagnosis of previous dengue infection
Abstract
Background: Dengue is an important mosquito-borne disease. There is currently only one licensed vaccine for dengue prevention. The vaccine provides higher efficacy in pre-vaccination dengue-seropositive persons but a higher risk of subsequent more severe dengue in dengue-seronegative persons. It is recommended that the dengue vaccine may be given in dengue-seropositive individuals or as mass vaccination without individual pre-vaccination screening in areas where the dengue seroprevalence is > 80% in children aged 9 years. We evaluated a dengue specific immunoglobulin G monoclonal antibody-based capture enzyme-linked immunosorbent assay (MAb-ELISA) in the diagnosis of previous dengue infection using serum samples from the cohort study in Ratchaburi Province, Thailand.
Methods: The MAb-ELISA was compared to 70% plaque reduction neutralization test (PRNT70) in 453 serum samples from children aged 3-11 years in Ratchaburi Province, Thailand.
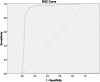
Results: The sensitivity and specificity of MAb-ELISA at the positive to negative (P/N) ratio cut-off level of > 3 were both 0.91 in the diagnosis of previous dengue infection, compared to PRNT70. The false positivity was mainly in Japanese encephalitis (JE) seropositive subjects.
Conclusions: This research provides evidence that MAb-ELISA is useful for dengue seroprevalence study and dengue pre-vaccination screening. JE seropositivity was the major cause of false positive result in the study population.
Keywords: Dengue; Enzyme-linked immunosorbent assay; Monoclonal antibody; Plaque reduction neutralization test.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. - DOI - PubMed
-
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M. Bouckenooghe a; CYD14 study group: clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. - DOI - PubMed
-
- Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Miranda Montoya MC, Cortés Supelano M, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F, CYD15 Study Group Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. - DOI - PubMed
-
- World Health Organization Strategic Advisory Group of Experts on immunization (SAGE): Summary of the April 2016 meeting of the Strategic Advisory Group of Experts on immunization (SAGE). http://www.who.int/immunization/sage/meetings/2016/april/SAGE_April_2016.... Accessed 19 Dec 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

