Treatment failure and hospital readmissions in severe COPD exacerbations treated with azithromycin versus placebo - a post-hoc analysis of the BACE randomized controlled trial
- PMID: 31665017
- PMCID: PMC6819655
- DOI: 10.1186/s12931-019-1208-6
Treatment failure and hospital readmissions in severe COPD exacerbations treated with azithromycin versus placebo - a post-hoc analysis of the BACE randomized controlled trial
Abstract
Background: In the BACE trial, a 3-month (3 m) intervention with azithromycin, initiated at the onset of an infectious COPD exacerbation requiring hospitalization, decreased the rate of a first treatment failure (TF); the composite of treatment intensification (TI), step-up in hospital care (SH) and mortality.
Objectives: (1) To investigate the intervention's effect on recurrent events, and (2) to identify clinical subgroups most likely to benefit, determined from the incidence rate of TF and hospital readmissions.
Methods: Enrolment criteria included the diagnosis of COPD, a smoking history of ≥10 pack-years and ≥ 1 exacerbation in the previous year. Rate ratio (RR) calculations, subgroup analyses and modelling of continuous variables using splines were based on a Poisson regression model, adjusted for exposure time.
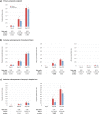
Results: Azithromycin significantly reduced TF by 24% within 3 m (RR = 0.76, 95%CI:0.59;0.97, p = 0.031) through a 50% reduction in SH (RR = 0.50, 95%CI:0.30;0.81, p = 0.006), which comprised of a 53% reduction in hospital readmissions (RR = 0.47, 95%CI:0.27;0.80; p = 0.007). A significant interaction between the intervention, CRP and blood eosinophil count at hospital admission was found, with azithromycin significantly reducing hospital readmissions in patients with high CRP (> 50 mg/L, RR = 0.18, 95%CI:0.05;0.60, p = 0.005), or low blood eosinophil count (<300cells/μL, RR = 0.33, 95%CI:0.17;0.64, p = 0.001). No differences were observed in treatment response by age, FEV1, CRP or blood eosinophil count in continuous analyses.
Conclusions: This post-hoc analysis of the BACE trial shows that azithromycin initiated at the onset of an infectious COPD exacerbation requiring hospitalization reduces the incidence rate of TF within 3 m by preventing hospital readmissions. In patients with high CRP or low blood eosinophil count at admission this treatment effect was more pronounced, suggesting a potential role for these biomarkers in guiding azithromycin therapy.
Trial registration: ClinicalTrials.gov number. NCT02135354 .
Keywords: CRP; Eosinophil count; Macrolide; Readmission; Recurrent event.
Conflict of interest statement
-KV is supported as a doctoral candidate by the Flemish Government Agency for Innovation by Science and Technology (Belgium).
-AB’s institute received consultancy fees from Boehringer-Ingelheim and UCB Pharma.
-KB’s institute received consultancy fees from Boehringer-Ingelheim and UCB Pharma.
-IG has nothing to disclose.
-NC has nothing to disclose.
-MG has nothing to disclose.
-JA has nothing to disclose.
-IKD has nothing to disclose.
-JLC has received speaker and consultancy fees from Boehringer-Ingelheim, AstraZeneca, Novartis, Chiesi and GlaxoSmithKline.
-EM has, within the last 5 years, received honoraria for lectures from Boehringer-Ingelheim, Chiesi and Novartis; he is a member of advisory boards for AstraZeneca, Chiesi, Boehringer-Ingelheim and Novartis.
-HS has received consultancy fees from Boehringer-Ingelheim and GlaxoSmithKline.
-CH has received speaker and consultancy fees from Boehringer-Ingelheim, Chiesi, AstraZeneca, GlaxoSmithKline and Novartis.
-SV has nothing to disclose.
-GMV has nothing to disclose.
-TT is vice president of the European Respiratory Society (2018–2019). His institute received speaker and consultancy fees from Boehringer-Ingelheim, AstraZeneca and Chiesi.
-VN has received speaker and consultancy fees from Boehringer-Ingelheim, AstraZeneca, Novartis, MSD, GlaxoSmithKline and Chiesi.
-GB has, within the last 5 years, received honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Pfizer, Teva, UCB Pharma and Zambon; he is a member of advisory boards for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Sanofi/Regeneron and Teva.
-WJ is supported as a senior clinical researcher by the Fund for Scientific Research Flanders (Belgium); and has received research funding, speaker and consultancy fees from Boehringer-Ingelheim, AstraZeneca, Novartis, Chiesi and GlaxoSmithKline. WJ is co-founder of ArtIQ.
Figures

References
-
- Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) Global strategy for the diagnosis, management, and prevention of COPD, 2018 report. 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

