Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018-2019 Season
- PMID: 31665373
- PMCID: PMC7325528
- DOI: 10.1093/infdis/jiz543
Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018-2019 Season
Abstract
Background: Increased illness due to antigenically drifted A(H3N2) clade 3C.3a influenza viruses prompted concerns about vaccine effectiveness (VE) and vaccine strain selection. We used US virologic surveillance and US Influenza Vaccine Effectiveness (Flu VE) Network data to evaluate consequences of this clade.
Methods: Distribution of influenza viruses was described using virologic surveillance data. The Flu VE Network enrolled ambulatory care patients aged ≥6 months with acute respiratory illness at 5 sites. Respiratory specimens were tested for influenza by means of reverse-transcriptase polymerase chain reaction and were sequenced. Using a test-negative design, we estimated VE, comparing the odds of influenza among vaccinated versus unvaccinated participants.
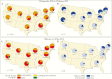
Results: During the 2018-2019 influenza season, A(H3N2) clade 3C.3a viruses caused an increasing proportion of influenza cases. Among 2763 Flu VE Network case patients, 1325 (48%) were infected with A(H1N1)pdm09 and 1350 (49%) with A(H3N2); clade 3C.3a accounted for 977 (93%) of 1054 sequenced A(H3N2) viruses. VE was 44% (95% confidence interval, 37%-51%) against A(H1N1)pdm09 and 9% (-4% to 20%) against A(H3N2); VE was 5% (-10% to 19%) against A(H3N2) clade 3C.3a viruses.
Conclusions: The predominance of A(H3N2) clade 3C.3a viruses during the latter part of the 2018-2019 season was associated with decreased VE, supporting the A(H3N2) vaccine component update for 2019-2020 northern hemisphere influenza vaccines.
Keywords: influenza; influenza vaccine; vaccine effectiveness.
Published by Oxford University Press for the Infectious Diseases Society of America 2019.
Figures
Comment in
-
Searching for Improved Flu Vaccines-The Time Is Now.J Infect Dis. 2020 Jan 1;221(1):1-4. doi: 10.1093/infdis/jiz545. J Infect Dis. 2020. PMID: 31665360 No abstract available.
References
-
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season. Wkly Epidemiol Rec 2019; 94:141–50.
-
- Process of influenza vaccine virus selection and development. http://apps.who.int/gb/pip/pdf_files/Fluvaccvirusselection.pdf. Accessed 17 April 2019.
-
- Recommended composition of influenza virus vaccines for use in the 2019- 2020 northern hemisphere influenza season 2019. https://www.who.int/influenza/vaccines/virus/recommendations/201902_reco.... Accessed 17 April 2019.
-
- Questions and answers: A(H3N2) component of the recommended composition of influenza virus vaccines for use in the northern hemisphere 2019–20 influenza season and development of candidate vaccine viruses for pandemic preparedness World Health Organization, 2019. https://www.who.int/influenza/vaccines/virus/recommendations/201902_qand.... Accessed 17 April 2019.
-
- Addendum to the recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season 2019. https://www.who.int/influenza/vaccines/virus/recommendations/201902_reco.... Accessed 17 April 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

