Molecular Diversification of the Seminal Fluid Proteome in a Recently Diverged Passerine Species Pair
- PMID: 31665510
- PMCID: PMC6993853
- DOI: 10.1093/molbev/msz235
Molecular Diversification of the Seminal Fluid Proteome in a Recently Diverged Passerine Species Pair
Abstract
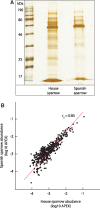
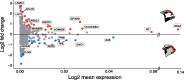
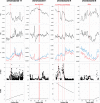
Seminal fluid proteins (SFPs) mediate an array of postmating reproductive processes that influence fertilization and fertility. As such, it is widely held that SFPs may contribute to postmating, prezygotic reproductive barriers between closely related taxa. We investigated seminal fluid (SF) diversification in a recently diverged passerine species pair (Passer domesticus and Passer hispaniolensis) using a combination of proteomic and comparative evolutionary genomic approaches. First, we characterized and compared the SF proteome of the two species, revealing consistencies with known aspects of SFP biology and function in other taxa, including the presence and diversification of proteins involved in immunity and sperm maturation. Second, using whole-genome resequencing data, we assessed patterns of genomic differentiation between house and Spanish sparrows. These analyses detected divergent selection on immunity-related SF genes and positive selective sweeps in regions containing a number of SF genes that also exhibited protein abundance diversification between species. Finally, we analyzed the molecular evolution of SFPs across 11 passerine species and found a significantly higher rate of positive selection in SFPs compared with the rest of the genome, as well as significant enrichments for functional pathways related to immunity in the set of positively selected SF genes. Our results suggest that selection on immunity pathways is an important determinant of passerine SF composition and evolution. Assessing the role of immunity genes in speciation in other recently diverged taxa should be prioritized given the potential role for immunity-related proteins in reproductive incompatibilities in Passer sparrows.
Keywords: cryptic female choice; fertility; immunity; positive selection; reproduction; selective sweep; sperm competition.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
References
-
- Ait Belkacem A, Gast O, Stuckas H, Canal D, LoValvo M, Giacalone G, Päckert M.. 2016. North African hybrid sparrows (Passer domesticus, P. hispaniolensis) back from oblivion—ecological segregation and asymmetric mitochondrial introgression between parental species. Ecol Evol. 6(15):5190–5206. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

